
|
|
Font Size:
|
||||
|
|
|
|
||||
STATISTICAL BRIEF #442:
Changes in Osteoporosis Medication Use and Expenditures among Women (Age ≥ 50), United States, 2000 to 2011
Highlights
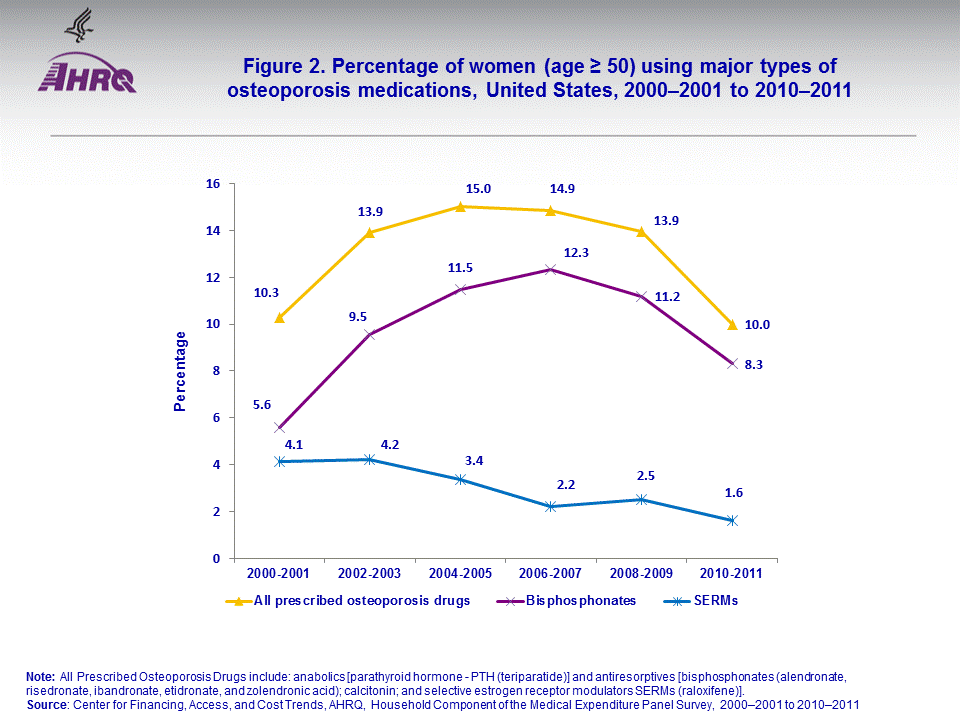
- Between 2000–2001 and 2010–2011, average annual percent of women (age ≥ 50) who reported using any selective estrogen receptor modulators (SERMs) decreased from 4.1 to 1.6 percent. However, the average annual percentage who reported using any bisphosphonates increased from 5.6 percent in 2000–2001 to 8.3 percent in 2010–2011.
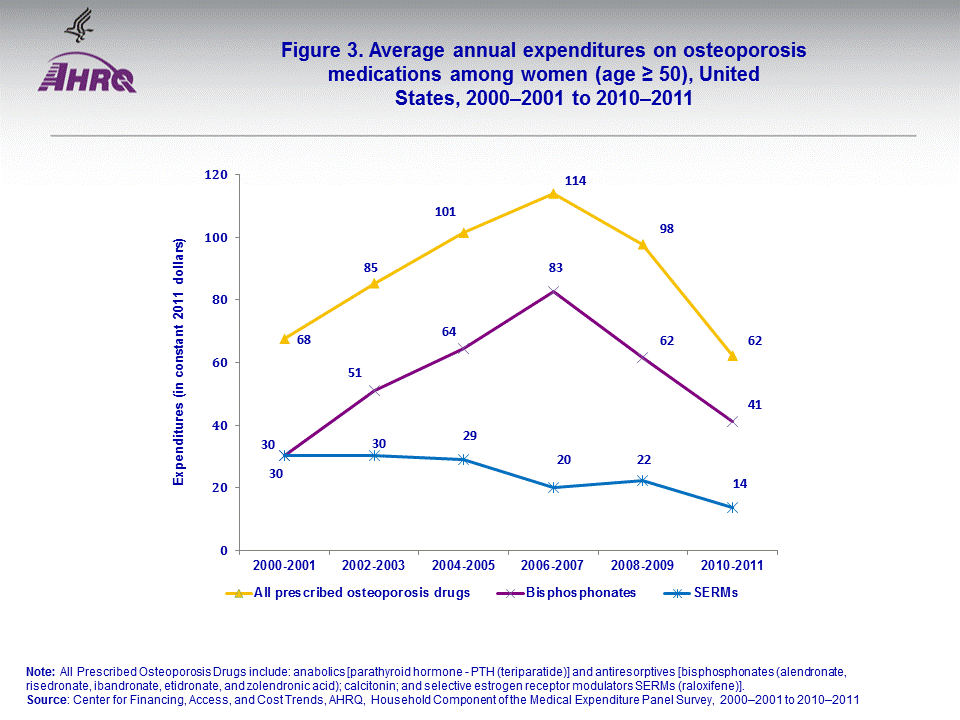
- Among women (age ≥ 50), average annual expenditures on SERMs decreased from $30 in 2000–2001 to $14 in 2010–2011. Average annual expenditures on bisphosphonates increased from $30 in 2000–2001 to $41 in 2010–2011.
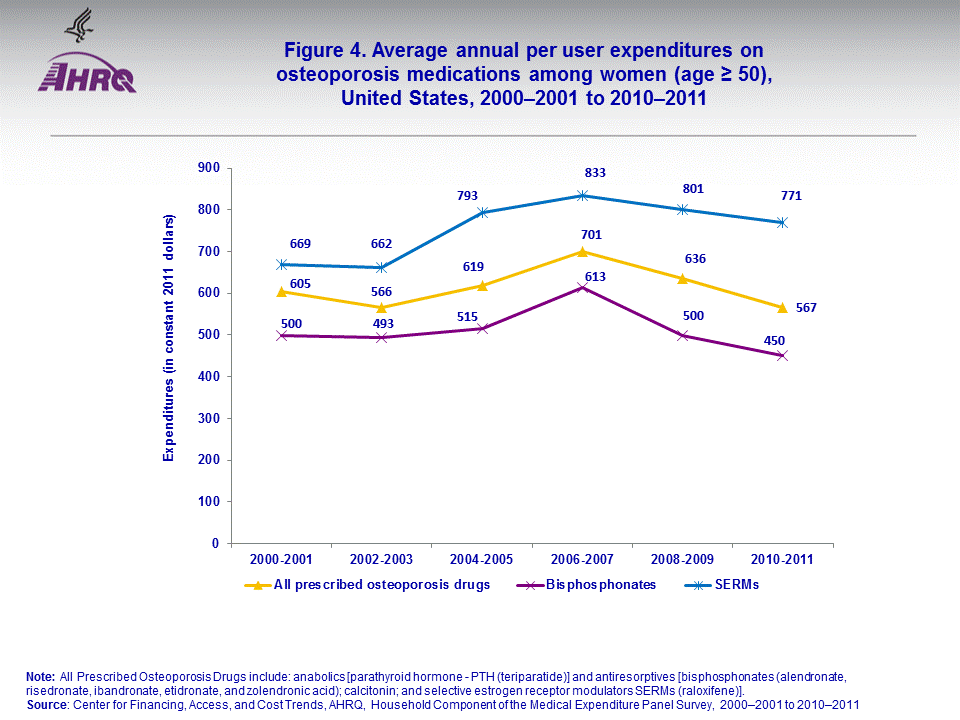
- Among women (age ≥ 50), annual expenditures per user on SERMs and bisphosphonates averaged $669 and $500 in 2000–2001, and $771 and $450 in 2010–2011.
Introduction
Osteoporosis, a disease that predominantly affects menopausal women, is characterized by compromised bone strength and increased risk of fracture, and if left untreated with pharmacological intervention can impose significant clinical and economic burden for patients and society. There are several pharmacological options that are approved by the U.S. Food and Drug Administration (FDA) for the prevention and/or treatment of osteoporosis.1,2,3This Statistical Brief examines changes in osteoporosis medication use and expenditures among women (age ≥ 50) in the United States using nationally representative data from the 2000 to 2011 Medical Expenditure Panel Survey (MEPS). The FDA-approved medications with indication for the prevention and/or treatment of osteoporosis examined in this Statistical Brief includes anabolics (i.e. parathyroid hormone—PTH) and antiresorptives (i.e., bisphosphonates, calcitonin, and selective estrogen receptor modulators—SERMs). Data from 2000–2001 to 2010–2011 are pooled to increase sample sizes and the precision of estimates; thus, results are presented as average annual estimates for these time periods. Expenditures for all years are expressed in constant 2011 U.S. dollars. All differences between estimates discussed in the text are statistically significant at the 0.05 level unless otherwise noted. This Brief begins by presenting results on trends in the use and expenditures for medications indicated for the prevention and/or treatment of osteoporosis.
Findings
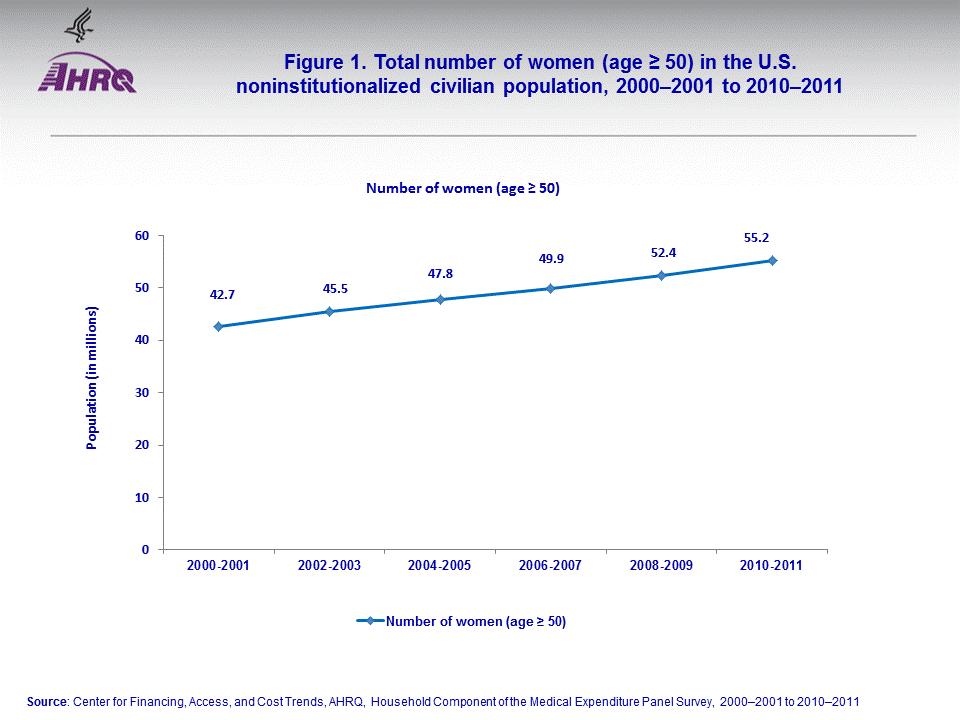
The number of women (age ≥ 50) in the U.S. noninstitutionalized civilian population increased from an average annual of 42.7 million in 2000–2001 to 55.2 million in 2010–2011 (figure 1).Among women (age ≥ 50), the average annual percent who reported using any SERMs declined from 4.1 percent in 2000–2001 to 1.6 percent in 2010–2011. During the same period, the average annual percentage who reported using bisphosphonates increased from 5.6 percent in 2000–2001 to 8.3 percent in 2010–2011 (figure 2).
Among women (age ≥ 50) using any prescription osteoporosis medication, the average annual expenditures for SERMs fell from $30 in 2000–2001 to $14 in 2010–2011 after adjustment for inflation. During the same period, the average annual expenditures for bisphosphonates increased 54 percent from $30 in 2000–2001 to $41 in 2010–2011 (figure 3).
Among women (age ≥ 50), the annual expenditures per user for SERMs and bisphosphonates averaged $669 and $500 in 2000–2001, and $771 and $450 in 2010–2011 (figure 4).
Data Source
The estimates presented in this Statistical Brief were derived from the MEPS Full Year Consolidated Data Files, the MEPS Medical Conditions Files, and the MEPS Prescribed Medicines Files for 2000 to 2011.Definitions
Osteoporosis medicationsEach drug that was listed as purchased or otherwise obtained in the MEPS Prescribed Medicines (PMED) Files was linked to the Multum Lexicon database, a product of Cerner Multum, Inc. The Multum drug name variable gives the active ingredient(s) in each drug and was used to identify the two major types of osteoporosis medications:—anabolics: parathyroid hormone—PTH (teriparatide)— antiresorptives: bisphosphonates (e.g., alendronate, risedronate, ibandronate, etidronate, pamidronate, and zolendronic acid); calcitonin; and selective estrogen receptor modulators SERMs (e.g., raloxifene). A class of approved osteoporosis medication, receptor activator of nuclear factor-kappa B (NFϰB) ligand (RANKL) inhibitor (denosumab), was not available in the MEPS during the analysis period. Information about indication and effectiveness of osteoporosis medications and their ability to reduce the risk of different fracture types can be found in the publications listed as footnotes 2 and 3.
Utilization
Indicator variables were created to identify persons who used each of the major classes of osteoporosis medications and their combinations during the year. For combination drugs, an adult was identified as having had each medication comprising the combination therapy. For example, if an adult had a combination drug that included both a bisphosphonate and calcium, then the adult was identified as having used each of these types of osteoporosis medications. Utilization estimates are presented as the percent of persons using each of the general types of osteoporosis medications, and each specific class of osteoporosis medication during the year.
Expenditures
Expenditures include all amounts paid for health care from any source including payments by individuals and their families and payments by private insurance, Medicaid, Medicare, and other types of insurance. For this Brief, all expenditures were adjusted to constant 2011 U.S. dollars using the Consumer Price Index for all urban consumers (CPI-U). All estimates presented are average annual estimates for the 2000–2001 to 2010–2011 periods.
About MEPS-HC
MEPS-HC is a nationally representative longitudinal survey that collects detailed information on health care utilization and expenditures, health insurance, and health status, as well as a wide variety of social, demographic, and economic characteristics for the U.S. civilian noninstitutionalized population. It is cosponsored by the Agency for Healthcare Research and Quality and the National Center for Health Statistics.For more information about MEPS, call the MEPS information coordinator at AHRQ (301-427-1656) or visit the MEPS Web site at http://www.meps.ahrq.gov/.
References
For a detailed description of the MEPS survey design, sample design, and methods used to minimize sources of nonsampling error, see the following publications:Cohen, J. Design and Methods of the Medical Expenditure Panel Survey Household Component. MEPS Methodology Report No. 1. AHCPR Pub. No. 97-0026. Rockville, MD: Agency for Health Care Policy and Research, 1997. http://www.meps.ahrq.gov/mepsweb/data_files/publications/mr1/mr1.shtml
Cohen, S. Sample Design of the 1996 Medical Expenditure Panel Survey Household Component. MEPS Methodology Report No. 2. AHCPR Pub. No. 97-0027. Rockville, MD: Agency for Health Care Policy and Research, 1997. http://www.meps.ahrq.gov/mepsweb/data_files/publications/mr2/mr2.shtml
Cohen, S. Design Strategies and Innovations in the Medical Expenditure Panel Survey. Medical Care, July 2003: 41(7) Supplement: III-5–III-12.
Ezzati-Rice, T.M., Rohde, F., Greenblatt, J. Sample Design of the Medical Expenditure Panel Survey Household Component, 1998–2007. Methodology Report No. 22. March 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://www.meps.ahrq.gov/mepsweb/data_files/publications/mr22/mr22.shtml
Suggested Citation
Sarpong, E. Changes in Osteoporosis Medication Use and Expenditures among Women (Age ≥ 50), United States, 2000 to 2011. Statistical Brief #442. June 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.meps.ahrq.gov/mepsweb/data_files/publications/st442/stat442.shtmlAHRQ welcomes questions and comments from readers of this publication who are interested in obtaining more information about access, cost, use, financing, and quality of health care in the United States. We also invite you to tell us how you are using this Statistical Brief and other MEPS data and tools and to share suggestions on how MEPS products might be enhanced to further meet your needs. Please email us at MEPSProjectDirector@ahrq.hhs.gov or send a letter to the address below:
Steven B. Cohen, PhD, Director
Center for Financing, Access, and Cost Trends
Agency for Healthcare Research and Quality
540 Gaither Road
Rockville, MD 20850
1 Note that FDA-approved medications that are indicated for the prevention and/or treatment of osteoporosis might be prescribed for reasons other than the prevention and/or treatment of osteoporosis.
2 National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Washington, D.C.: National Osteoporosis Foundation; 2010. Available at http://nof.org/files/nof/public/content/file/344/upload/159.pdf.
3 Crandall CJ, Newberry SJ, Gellad WG, Diamant A, Lim YW, Suttorp M, Motala A, Ewing B, Roth B, Timmer M, Shanman R, Shekelle PG. Treatment to Prevent Fractures in Men and Women with Low Bone Density or Osteoporosis: Update of a 2007 Report. Comparative Effectiveness Review No. 53. (Prepared by Southern California Evidence-based Practice Center under Contract No. HHSA-290-2007-10062-I.) Rockville, MD: Agency for Healthcare Research and Quality; March 2012. Available at http://www.effectivehealthcare.ahrq.gov/ehc/products/160/1007/CER53_LowBoneDensity_FinalReport_20120823.pdf.
 |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||


