|
August 2015
Agency for Healthcare Research and Quality
Center for Financing, Access, and Cost Trends
540 Gaither Road
Rockville, MD 20850
(301) 427-1406
Table of Contents
A. Data Use Agreement
B. Background
1.0 Household Component (HC)
2.0 Medical Provider Component (MPC)
3.0 Survey Management and Data Collection
C. Technical Information
1.0 General Information
2.0 Data File Information
2.1 Codebook Structure
2.2 Reserved Codes
2.3 Codebook Format
2.4 Variable Naming Conventions
2.4.1 General
2.4.2 Expenditure and Source of Payment Variables
2.5 Data Collection
2.5.1 Methodology for Collecting Household-Reported Variables
2.5.2 Methodology for Collecting Pharmacy-Reported Variables
2.6 File Contents
2.6.1 Survey Administration Variables
2.6.1.1 Person Identifier Variables (DUID, PID, DUPERSID)
2.6.1.2 Record Identifier Variables (RXRECIDX, LINKIDX, DRUGIDX)
2.6.1.3 Panel Variable (PANEL)
2.6.1.4 Round Variable (PURCHRD)
2.6.2 Characteristics of Prescribed Medicine Events
2.6.2.1 Date When Prescribed Medicine Was First Taken (RXBEGMM-RXBEGYRX)
2.6.2.2 Prescribed Medicine Attributes (RXNAME-RXDAYSUP)
2.6.2.3 Type of Pharmacy (PHARTP1-PHARTP8)
2.6.2.4 Analytic Flag Variables (RXFLG-INPCFLG)
2.6.2.5 Free Sample Variable (SAMPLE)
2.6.2.6 Clinical Classification Codes (RXCCC1X-RXCCC3X)
2.6.3 Multum Lexicon Variables from Cerner Multum, Inc.
2.6.4 Expenditure Variables (RXSF13X-RXXP13X)
2.6.4.1 Definition of Expenditures
2.6.4.2 Sources of Payment
3.0 Sample Weight (PERWT13F)
3.1 Overview
3.2 Details on Person Weight Construction
3.2.1 MEPS Panel 17 Weight Development Process
3.2.2 MEPS Panel 18 Weight Development Process
3.2.3 The Final Weight for 2013
3.3 Coverage
3.4 Using MEPS Data for Trend Analysis
4.0 General Data Editing and Imputation Methodology
4.1 Rounding
4.2 Edited/Imputed Expenditure Variables (RXSF13X-RXXP13X)
5.0 Strategies for Estimation
5.1 Developing Event-Level Estimates
5.2 Person-Based Estimates for Prescribed Medicine Purchases
5.3 Variables with Missing Values
5.4 Variance Estimation (VARSTR, VARPSU)
6.0 Merging/Linking MEPS Data Files
6.1 Linking to the Person-Level File
6.2 Linking to the Medical Conditions File
6.3 Longitudinal Analysis
References
D. Variable-Source Crosswalk
Appendix 1: Definitions for RXFORM, Dosage Form
Appendix 2: Definitions for RXFRMUNT, Quantity Unit of Medication
Appendix 3: Definitions for RXSTRUNT, Unit of Medication
Appendix 4: Definitions of Therapeutic Class Code
Individual identifiers have been removed from the
micro-data contained in these files. Nevertheless, under sections 308 (d) and
903 (c) of the Public Health Service Act (42 U.S.C. 242m and 42 U.S.C. 299 a-1),
data collected by the Agency for Healthcare Research and Quality (AHRQ) and/or
the National Center for Health Statistics (NCHS) may not be used for any purpose
other than for the purpose for which they were supplied; any effort to determine
the identity of any reported cases is prohibited by law.
Therefore in accordance with the above referenced
Federal Statute, it is understood that:
- No one is to use the data in this data set in any way except
for statistical reporting and analysis; and
- If the identity of any person or establishment should be
discovered inadvertently, then (a) no use will be made of this
knowledge, (b) the Director Office of Management AHRQ will be
advised of this incident, (c) the information that would
identify any individual or establishment will be safeguarded or
destroyed, as requested by AHRQ, and (d) no one else will be
informed of the discovered identity; and
- No one will attempt to link this data set with individually
identifiable records from any data sets other than the Medical
Expenditure Panel Survey or the National Health Interview
Survey.
By using these data you signify your agreement to
comply with the above stated statutorily based requirements with the knowledge
that deliberately making a false statement in any matter within the jurisdiction
of any department or agency of the Federal Government violates Title 18 part 1
Chapter 47 Section 1001 and is punishable by a fine of up to $10,000 or up to 5
years in prison.
The Agency for Healthcare Research and Quality
requests that users cite AHRQ and the Medical Expenditure Panel Survey as the
data source in any publications or research based upon these data.
Return To Table Of Contents
TThe Medical Expenditure Panel Survey (MEPS) provides
nationally representative estimates of health care use, expenditures, sources of
payment, and health insurance coverage for the U.S. civilian
non-institutionalized population. The MEPS Household Component (HC) also
provides estimates of respondents' health status, demographic and socio-economic
characteristics, employment, access to care, and satisfaction with health care.
Estimates can be produced for individuals, families, and selected population
subgroups. The panel design of the survey, which includes 5 Rounds of interviews
covering 2 full calendar years, provides data for examining person level changes
in selected variables such as expenditures, health insurance coverage, and
health status. Using computer assisted personal interviewing (CAPI) technology,
information about each household member is collected, and the survey builds on
this information from interview to interview. All data for a sampled household
are reported by a single household respondent.
The MEPS-HC was initiated in 1996. Each year a new
panel of households is selected. Because the data collected are comparable to
those from earlier medical expenditure surveys conducted in 1977 and 1987, it is
possible to analyze long-term trends. Each annual MEPS-HC sample size is about
15,000 households. Data can be analyzed at either the person or event level.
Data must be weighted to produce national estimates.
The set of households selected for each panel of the
MEPS HC is a subsample of households participating in the previous year's
National Health Interview Survey (NHIS) conducted by the National Center for
Health Statistics. The NHIS sampling frame provides a nationally representative
sample of the U.S. civilian non-institutionalized population and reflects an
oversample of Blacks and Hispanics. In 2006, the NHIS implemented a new sample
design, which included Asian persons in addition to households with Black and
Hispanic persons in the oversampling of minority populations. MEPS oversamples
additional policy relevant sub-groups such as Asians and low income households.
The linkage of the MEPS to the previous year's NHIS provides additional data for
longitudinal analytic purposes.
Return To Table Of Contents
Upon completion of the household CAPI interview and
obtaining permission from the household survey respondents, a sample of medical
providers are contacted by telephone to obtain information that household
respondents can not accurately provide. This part of the MEPS is called the
Medical Provider Component (MPC) and information is collected on dates of visit,
diagnosis and procedure codes, charges and payments. The Pharmacy Component
(PC), a subcomponent of the MPC, does not collect charges or diagnosis and
procedure codes but does collect drug detail information, including National
Drug Code (NDC) and medicine name, as well as date filled and sources and
amounts of payment. The MPC is not designed to yield national estimates. It is
primarily used as an imputation source to supplement/replace
household-reported expenditure information.
Return To Table Of Contents
MEPS HC and MPC data are collected under the authority
of the Public Health Service Act. Data are collected under contract with Westat,
Inc. (MEPS HC) and Research Triangle Institute (MEPS MPC). Data sets and summary
statistics are edited and published in accordance with the confidentiality
provisions of the Public Health Service Act and the Privacy Act. The National
Center for Health statistics (NCHS) provides consultation and technical
assistance.
As soon as data collection and editing are completed,
the MEPS survey data are released to the public in staged releases of summary
reports, micro data files, and tables via the MEPS web site:
meps.ahrq.gov . Selected data can be
analyzed through MEPSnet, an on-line interactive tool designed to give data
users the capability to statistically analyze MEPS data in a menu-driven
environment.
Additional information on MEPS is available from the
MEPS project manager or the MEPS public use data manager at the Center for
Financing Access and Cost Trends, Agency for Healthcare Research and Quality,
540 Gaither Road, Rockville, MD 20850 (301-427-1406).
Return To Table Of Contents
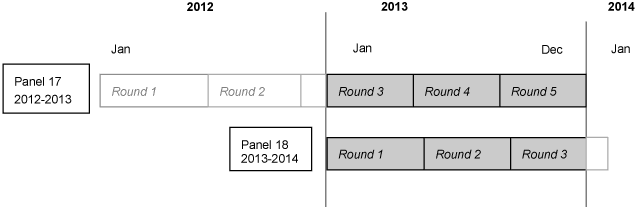
This documentation describes one in a series of public
use event files from the 2013 Medical Expenditure Panel Survey (MEPS) Household
Component (HC) and Medical Provider Component (MPC). Released as an ASCII data
file (with related SAS, SPSS, and Stata programming statements) and SAS
transport file, the 2013 Prescribed Medicines public use file provides detailed
information on household-reported prescribed medicines for a nationally
representative sample of the civilian noninstitutionalized population of the
United States. Data from the Prescribed Medicines event file can be used to make
estimates of prescribed medicine utilization and expenditures for calendar year
2013. The file contains 69 variables and has a logical record length of 584 with
an additional 2-byte carriage return/line feed at the end of each record. As
illustrated below, this file consists of MEPS survey data obtained in the 2013
portion of Round 3 and Rounds 4 and 5 for Panel 17, as well as Rounds 1, 2 and
the 2013 portion of Round 3 for Panel 18 (i.e., the rounds for the MEPS panels
covering calendar year 2013).
Each record on this event file represents a unique
prescribed medicine event; that is, a prescribed medicine reported as being
purchased by the household respondent. In addition to expenditures related to
the prescribed medicine, each record contains household-reported characteristics
and medical conditions associated with the prescribed medicine.
Data from this event file can be merged with other
2013 MEPS-HC data files, for purposes of appending person characteristics such
as demographic or health insurance coverage to each prescribed medicine record.
Counts of prescribed medicine utilization are based
entirely on household reports. Information from the Pharmacy Component (PC)
(within the MEPS-MPC, see Section B 2.0 for more details on the MPC) was used to
provide expenditure and payment data, as well as details of the medication
(e.g., strength, quantity, etc.).
The file can be used to construct summary variables of
expenditures, sources of payment, and other aspects of utilization of prescribed
medicines. Aggregate annual person-level information on the use of prescribed
medicines and other health services use is provided on the 2013 Full Year Consolidated Data File,
where each record represents a MEPS sampled person.
The following documentation offers a brief overview of
the types and levels of data provided and the content and structure of the files
and the codebook. It contains the following sections:
- Data File Information
- Sample Weight
- General Data Editing and Imputation Methodology
- Strategies for Estimation
- Merging/Linking MEPS Data Files
- References
- Variable - Source Crosswalk
For more information on MEPS HC survey design see T.
Ezzati-Rice, et al. (1998-2007) and S. Cohen, 1996. For information on the MEPS MPC design, see S. Cohen, 1998.
A copy of the survey instrument used to collect the information on this file
is available on the MEPS Web site at the following address:
meps.ahrq.gov.
Return To Table Of Contents
The 2013 Prescribed Medicines public use data set
contains 327,557 prescribed medicine records. Each record represents one
household-reported prescribed medicine that was purchased during calendar year
2013. Of the 327,557 prescribed medicine records, 321,552 records are associated
with persons having a positive person-level weight (PERWT13F). The persons
represented on this file had to meet either criterion a) or b) below:
- Be classified as a key in-scope person who
responded for his or her entire period of 2013 eligibility (i.e., persons with a
positive 2013 full-year person-level sampling weight (PERWT13F > 0)), or
- Be an eligible member of a family all of whose key
in-scope members have a positive person-level weight (PERWT13F > 0). (Such a
family consists of all persons with the same value for FAMIDYR.) That is, the
person must have a positive full-year family-level weight (FAMWT13F > 0). Note that FAMIDYR and FAMWT13F are
variables on the 2013 Full Year Consolidated Data File.
Persons with no prescribed medicine use for 2013 are
not included on this file (but are represented on MEPS person-level files). A
codebook for the data file is provided (in file H160acb.pdf).
This file includes prescribed medicine records for all
household members who resided in eligible responding households and for whom at
least one prescribed medicine was reported. Only prescribed medicines that were
purchased in calendar year 2013 are represented on this file. This file includes
prescribed medicines identified in the Prescribed Medicines (PM) section of the
HC survey instrument, as well as those prescribed medicines identified in
association with other medical events. Each record on this file represents a
single acquisition of a prescribed medicine reported by household respondents.
Some household members may have multiple acquisitions of prescribed medicines
and thus will be represented in multiple records on this file. Other household
members may have no reported acquisitions of prescribed medicines and thus will
have no records on this file.
When diabetic supplies, such as syringes and insulin,
were mentioned in the Other Medical Expenses (OM) section of the MEPS-HC, the
interviewer was directed to collect information on these items in the Prescribed
Medicines section of the MEPS questionnaire. The respondent was also asked the
questions in the Charge Payment (CP) section of the HC. To the extent that these
items are purchased without a prescription, they represent a non-prescription
addition to the MEPS prescription drug expenditure and utilization data.
Although these items may be purchased without a prescription, a prescription
purchase may be required to obtain third party payments. Analysts are free to
code and define diabetic supply/equipment and insulin events utilizing their own
coding mechanism. If desired, this would enable analysts to subset the
Prescribed Medicines file to exclude these types of events.
It should also be noted that refills are included on
this file. The HC obtains information on the name of the prescribed medicine and
the number of times the medicine was obtained. The data collection design for
the HC does not allow separate records to be created for multiple acquisitions
of the same prescribed medicine. However, in the PC, each original purchase, as
well as any refill, is considered a unique prescribed medicine event. Therefore,
for the purposes of editing, imputation, and analysis, all records in the HC
were “unfolded” to create separate records for each original purchase and each
refill. Please note that for multiple acquisitions of the same drug, MEPS did
not collect information in the HC to distinguish between the original purchase
and refills. The survey only collected data on the number of times a prescribed
medicine was acquired during a round. In some cases, all purchases may have been
refills of an original purchase in a prior round or prior to the survey year.
The file also includes a variable, SAMPLE, which indicates whether or not the
household reported receiving a free sample of that drug in that round. (To
obtain more details on free samples, please see Section 2.6.2.5.)
Each record on this file includes the following: an
identifier for each unique prescribed medicine; detailed characteristics
associated with the event (e.g., national drug code (NDC), medicine name,
selected Multum Lexicon variables [see Section 2.6.3 for more information on the
Multum Lexicon variables included on this file], etc.); conditions, if any,
associated with the medicine; the date on which the person first used the
medicine; total expenditure and sources of payments; types of pharmacies that
filled the household’s prescriptions; whether the prescription is one of which
the household received a free sample during the round; and a full-year
person-level weight.
Data from this file can be merged with previously
released MEPS-HC person-level data using the unique person identifier, DUPERSID,
to append person characteristics such as demographic or health insurance
coverage to each record. Data from this file can also be merged with the 2013
Full Year Consolidated Data File to estimate expenditures for persons with
prescribed medicines. The Prescribed Medicines event file can also be linked to
the MEPS 2013 Medical Conditions File and additional MEPS 2013 event files.
Please see the 2013 Appendix File for details on how to link MEPS data files.
Return To Table Of Contents
For most variables on the file, both weighted and
unweighted frequencies are provided. The exceptions to this are weight variables
and variance estimation variables. Only unweighted frequencies of these
variables are included in the accompanying codebook file. See the Weights
Variables list in section D, Variable-Source Crosswalk. The codebook and data
file sequence list variables in the following order:
- Unique person identifiers
- Unique prescribed medicine identifiers
- Other survey administration variables
- Prescribed medicine characteristics variables
- Clinical Classification Software codes for medical conditions
- Multum Lexicon variables
- Expenditure variables
- Weight and variance estimation variables
Return To Table Of Contents
The following reserved code values are used:
| Value |
Definition |
| -1 INAPPLICABLE |
Question was not asked due to skip pattern |
| -7 REFUSED |
Question was asked and respondent refused to answer question |
| -8 DK |
Question was asked and respondent did not know answer |
| -9 NOT ASCERTAINED |
Interviewer did not record the data |
| -14 NOT YET TAKEN/USED |
Respondent answered that the medicine has not yet been used |
Generally, values of -1, -7, -8 and -9 have not been
edited on this file. However, this is not true if a
prescription drug name was determined to be a confidentiality risk. In these instances, the
corresponding NDC was replaced with -9, the Multum Lexicon therapeutic class
replaced the drug name determined to be a confidentiality risk, and RXDRGNAM was set to -9.
The values of -1 and -9 can be edited by analysts by following the skip patterns in the
questionnaire. The value -14 was a valid value only for the variable
representing the year the household member first used the medicine (RXBEGYRX).
RXBEGYRX = -14 means that when the interviewer asked the respondent the year the
household member first started using the medicine, he/she responded that the
household member had not yet started using the medicine (See section C,
2.6.2.1).
A copy of the Household Component questionnaire can be
found at
meps.ahrq.gov/survey_comp/survey_questionnaires.jsp
by selecting Prescribed Medicines (PM) from the questionnaire section.
Return To Table Of Contents
The codebook describes an ASCII data set (although the
data are also being provided in a SAS transport file). The following codebook
items are provided for each variable:
| Identifier |
Description |
| Name |
Variable name (maximum of 8 characters) |
| Description |
Variable descriptor (maximum 40 characters) |
| Format |
Number of bytes |
| Type |
Type of data: numeric (indicated by NUM) or character (indicated by CHAR) |
| Start |
Beginning column position of variable in record |
| End |
Ending column position of variable in record |
Return To Table Of Contents
In general, variable names reflect the content of the
variable, with an eight-character limitation. Generally, all imputed/edited
variables end with an “X.”
Variables contained on this file were derived from the
HC questionnaire itself, the MPC data collection instrument, the CAPI, or from
the Multum Lexicon database from Cerner Multum, Inc. The source of each variable
is identified in Section D, entitled “Variable-Source Crosswalk.” Sources for
each variable are indicated in one of five ways:
- Variables which are derived from CAPI or assigned in
sampling are so indicated as “CAPI derived” or “Assigned in
sampling,” respectively;
- Variables which come from one or more specific questions
have those numbers and the questionnaire section indicated in
the “Source” column;
- Variables constructed from multiple questions using complex
algorithms are labeled “Constructed” in the “Source” column;
- Variables which have been imputed are so indicated; and
- Variables derived from the Multum Lexicon database are so
indicated.
Return To Table Of Contents
Only imputed/edited versions of the expenditure
variables are provided on the file. Expenditure variables on this event file
follow a standard naming convention and are 7 characters in length.
The 12 source of payment variables and one sum of
payments variable are named consistently in the following way:
The first two characters indicate the type of event:
IP - inpatient stay
ER - emergency room visit
HH - home health visit
OM - other medical equipment
OB - office-based visit
OP - outpatient visit
DV - dental visit
RX - prescribed medicine
In the case of the source of payment variables, the
third and fourth characters indicate:
SF - self or family
MR - Medicare
MD - Medicaid
PV - private insurance
VA - Veterans Administration/CHAMPVA
TR - TRICARE OU - other public
OF - other federal government
SL - state/local government
WC - Workers’ Compensation
OT - other insurance
OR - other private
XP - sum of payments
The fifth and sixth characters indicate the year (13).
The seventh character, “X” , indicates the variable is edited/imputed.
For example, RXSF13X is the edited/imputed amount paid
by self or family for the 2013 prescribed medicine expenditure.
Return To Table Of Contents
Data regarding prescription drugs were obtained
through the HC questionnaire and a pharmacy follow-back component (within
the Medical Provider Component).
Return To Table Of Contents
During each round of the MEPS-HC, respondents were
asked to supply the name of any prescribed medicine they or their family members
purchased or otherwise obtained during that round. For each medicine in each
round, the following information was collected: whether any free samples of the
medicine were received; the name(s) of any health problems the medicine was
prescribed for; the number of times the prescription medicine was obtained or
purchased; the year and month in which the person first used the
medicine; and a list of the names, addresses, and types of pharmacies that
filled the household’s prescriptions. In the HC, respondents were asked
if they send in claim forms for their prescriptions or if their pharmacy
providers do this automatically for them at the point of purchase. For those who
said their pharmacy providers automatically send in claims for them at the point
of purchase, charge and payment information was collected in the pharmacy
follow-back component (unless the purchase was an insulin or diabetic
supply/equipment event that was mentioned in the household component; see
Section 4.0 for details). However, charge and payment information was collected
in the HC for those who said they send in their own prescription claim forms,
because it is thought that payments by private third-party payers for those who
filed their own claim forms for prescription purchases would not be available
from pharmacies. Uninsured persons were treated in the same manner as those
whose pharmacies filed their prescription claims at the point of purchase.
Persons who said they did not know if they sent in their own prescription claim
forms were treated as those who said they did send in their own prescription
claim forms.
In consultation with an industry expert, outlier
values for the number of times a household reported purchasing or otherwise
obtaining a prescription drug in a particular round were determined by comparing
the number of days a person was in the round to the number of times the person
was reported to have obtained the drug in the round. For these events, a new
value for the number of times a drug was purchased or otherwise obtained by a
person in a round was imputed. In addition, for rounds in which a household
respondent did not know/remember the number of times a certain prescribed
medicine was purchased or otherwise obtained, the number of fills or refills was
imputed.
For those rounds that spanned two years, drugs
mentioned in that round were allocated between the years based on the number of
times the respondent said the drug was purchased in the respective year, the
year the person started taking the drug, the length of the person’s round, the
dates of the person’s round, and the number of drugs for that person in the
round. In addition, a “folded” version of the PC on a drug level, as opposed to
an acquisition level, was used for these types of events to assist in
determining how many acquisitions of the drug should be allocated between the
years.
Return To Table Of Contents
If the household member with the prescription gave
written permission to release his or her pharmacy records, pharmacy providers
identified by the household were contacted by telephone for the pharmacy
follow-back component. Following an initial telephone contact, the signed
permission forms and materials explaining the study were faxed (or mailed) to
cooperating pharmacy providers. The materials informed the providers of all
persons participating in the survey who had prescriptions filled at their place
of business and requested a computerized printout of all prescriptions filled
for each person. Pharmacies can choose to report information in computer
assisted telephone interviews (CATI). The CATI instrument was also used to enter
information from printouts. For each medication listed, the following
information was requested: date filled; national drug code (NDC); medication
name; strength of medicine (amount and unit); quantity (package size/amount
dispensed); and payments by source. When an NDC was provided, often the drug
name and other drug characteristics were obtained from secondary proprietary
data sources.
Return To Table Of Contents
The dwelling unit ID (DUID) is a five-digit random
number assigned after the case was sampled for MEPS. The three-digit person
number (PID) uniquely identifies each person within the dwelling unit. The
eight-character variable DUPERSID uniquely identifies each person represented on
the file and is the combination of the variables DUID and PID. For detailed
information on dwelling units and families, please refer to the documentation
for the 2013 Full Year Population Characteristics File.
Return To Table Of Contents
The variable RXRECIDX uniquely identifies each record
on the file. This 15-character variable comprises the following components:
prescribed medicine drug-round-level identifier generated through the HC
(positions 1-12) + enumeration number (positions 13-15). The prescribed medicine
drug-round-level ID generated through the HC (positions 1-12) can be used to
link a prescribed medicine event to the conditions file and to other event
files, via link files, and is provided on this file as the variable LINKIDX. For
more details on linking, please refer to Section 6.2 and to the 2013 Appendix
File. The prescribed medicine drug-level ID generated through the HC, DRUGIDX,
can be used to link drugs across rounds. DRUGIDX was first added to the file for
2009; for 1996 through 2008, the RXNDC linked drugs across rounds.
The following hypothetical example illustrates the
structure of these ID variables. This example illustrates a person in Rounds 1
and 2 of the household interview who reported having purchased Amoxicillin three
times. The following example shows three acquisition-level records, all having
the same DRUGIDX (00002026002), for one person (DUPERSID=00002026) in two
rounds. Generally, within a round, one NDC is associated with a prescribed
medicine event because matching was performed at a drug level, as opposed to an
acquisition level. The LINKIDX (000020260083) remains the same for both records
in Round 1 but varies across rounds. The RXRECIDX (000020260083001,
000020260083002, 000020260103001) differs for all three records.
The codebook describes an ASCII data set (although the
data are also being provided in a SAS transport file). The following codebook
items are provided for each variable:
| DUPERSID |
PURCHRD |
RXRECIDX |
LINKIDX |
DRUGIDX |
RXNDC |
| 00002026 |
1 |
000020260083001 |
000020260083 |
00002026002 |
00093310905 |
| 00002026 |
1 |
000020260083002 |
000020260083 |
00002026002 |
00093310905 |
| 00002026 |
2 |
000020260103001 |
000020260103 |
00002026002 |
00003010955 |
There can be multiple RXNDCs for a LINKIDX. All the
acquisitions in the LINKIDX represent the same drug (active ingredients), but
the RXNDCs may represent different manufacturers. (For more details on matching, please see Section 4.0).
Return To Table Of Contents
PANEL is a constructed variable used to specify the
panel number for the person. Panel will indicate either Panel 17 or Panel 18 for
each person on the file. Panel 17 is the panel that started in 2012, and Panel
18 is the panel that started in 2013.
Return To Table Of Contents
The variable PURCHRD indicates the round in which the
prescribed medicine was purchased and takes on the value of 1, 2, 3, 4, or 5.
Rounds 3, 4, and 5 are associated with MEPS survey data collection from Panel
17. Similarly, Rounds 1, 2, and 3 are associated with data collected from Panel
18.
Return To Table Of Contents
There are two variables which indicate when a
prescribed medicine was first taken (used), as reported by the household
respondent. They are the following: RXBEGMM denotes the month in which a person
first started taking a medication, and RXBEGYRX reflects the year in which a
person first started taking a medicine. These “first taken” questions are only
asked the first time a prescription is mentioned by the household respondent.
These questions are not asked about refills of the prescription in subsequent
rounds. Values are carried forward from prior rounds for all medications. Users should also note that the value -14
(not yet used or taken) is not relevant for refills. The variable DRUGIDX (see
Section 2.6.1.2) can be used to determine whether a medication was reported in a
prior round. For purposes of confidentiality, RXBEGYRX was bottom-coded at 1928,
consistent with top-coding of the age variables on the 2013 Full Year Population
Characteristics Public Use File (HC-157).
Return To Table Of Contents
For each prescribed medicine included on this file,
several data items collected describe in detail the medication obtained or
purchased. These data items are the following:
- Medication name - pharmacy reported (RXNAME)
- Medication name – Multum Lexicon (RXDRGNAM)
- National drug code (RXNDC)
- Quantity of the prescribed medicine dispensed (RXQUANTY),
e.g., number of tablets in the prescription
- Form of the prescribed medicine (RXFORM), e.g., powder
- Unit of measurement for form of Rx/prescribed medicine
(RXFRMUNT), e.g., oz
- Strength of the dose of the prescribed medicine (RXSTRENG),
e.g., 10
- Unit of measurement for the strength of the dose of the
prescribed medicine (RXSTRUNT), e.g., gm
- Days supplied (RXDAYSUP)
Days supplied was first collected and released to the
public on the 2010 Prescribed Medicines file. Many pharmacies did not provide
this information, and imputation was not attempted in these cases. A value of
999 indicates the medication is to be taken as needed. No edits were implemented
to impose consistency between the quantity and days supplied, and no edits were
implemented for very high values.
The 2013 file contains multiple values of RXFORM and
RXFRMUNT not found in Prescribed Medicines files in prior years. There was no
reconciliation of inconsistencies or duplication between RXFORM and RXFRMUNT.
Please refer to Appendices 1, 2, and 3 for definitions for RXFORM, RXFRMUNT, and
RXSTRUNT abbreviations, codes and symbols. Please refer to Appendix 4 for
therapeutic class code definitions.
The national drug code (NDC) is an 11-digit code. The
first 5 digits indicate the manufacturer of the prescribed medicine. The next 4
digits indicate the form and strength of the prescription, and the last 2 digits
indicate the package size from which the prescription was dispensed. NDC values
were imputed from a proprietary database to certain PC prescriptions because the
NDC reported by the pharmacy provider was not valid. These records are
identified by RXFLG = 3.
For the years 1996-2004, AHRQ’s licensing agreement
for the proprietary database precluded the release of the imputed NDC values to
the public, so for these prescriptions, the household-reported name of the
prescription (RXHHNAME) and the original NDC (RXNDC) and prescription name
(RXNAME) reported by the pharmacy were provided on the file to allow users to do
their own imputation. In addition, for the years 1996-2004, the imputed NDC
values for the RXFLG = 3 cases could be accessed through the MEPS Data Center.
For those events not falling into the RXFLG = 3 category, the reserve code
(-13) was assigned to the household-reported medication name (RXHHNAME). The
household-reported name of the prescription (RXHHNAME) is no longer provided on
this file; however, this variable may be accessed through the MEPS Data Center
as can the original pharmacy-reported name and NDC. For information on accessing
data through the MEPS Data Center, see the Data Center section of the MEPS Web
site at:
meps.ahrq.gov/data_stats/onsite_datacenter.jsp.
Beginning with the 2013 data, the variable RXDRGNAM is included on the file.
This drug name is the generic name of the drug most commonly used by prescribing
physicians. It is supplied by the Multum Lexicon database.
Imputed data on this event file, unlike other MEPS
event files, may still have missing data. This is because imputed data on this
file are imputed from the PC or from a proprietary database. These sources did
not always include complete information for each variable but did include an
NDC, which would typically enable an analyst to obtain any missing data items.
For example, although there are a substantial number of missing values for the
strength of the prescription that were not supplied by the pharmacist, these
missing values were not imputed because this information is embedded in the NDC.
Return To Table Of Contents
Household respondents were asked to list the type of
pharmacy from which household members purchased their medications. A respondent
could list multiple pharmacies associated with each member’s prescriptions in a
given round or over the course of all rounds combined covering the survey year.
All household-reported pharmacies are provided on this file, but there is no
link in the survey or in the data file enabling users to know the type of
pharmacy from which a specific prescription was obtained if multiple pharmacies
are listed. The variables PHARTP1 through PHARTP8 identify the types of pharmacy
providers from which the person’s prescribed medicines were purchased. The
possible types of pharmacies include the following: (1) mail-order, (2) another
store, (3) HMO/clinic/hospital, (4) drug store, and (5) on-line. A -1 value for
PHARTPn indicates that the household did not report “nth” pharmacy.
Return To Table Of Contents
There are five flag variables included on this file
(RXFLG, IMPFLAG, PCIMPFLG, CLMOMFLG, and INPCFLG).
RXFLG indicates whether or not there was any
imputation performed on this record for the NDC variable, and if imputed, from
what source the NDC was imputed. If no imputation was performed, RXFLG = 1. If
the imputation source was another PC record, RXFLG = 2. Similarly, if the
imputation source was a secondary, proprietary database and not the PC database,
RXFLG = 3.
IMPFLAG indicates the method of creating the
expenditure data: IMPFLAG = 1 indicates complete HC data, IMPFLAG = 2 indicates
complete PC data, IMPFLAG = 3 indicates HC and PC data, IMPFLAG = 4 indicates
fully imputed data, and IMPFLAG = 5 indicates partially imputed data.
PCIMPFLG indicates the type of match between a
household-reported event and a PC-reported event. PCIMPFLG = 1 indicates an
exact match for a specific event for a person between the PC and the HC.
PCIMPFLG = 2 indicates not an exact match between the PC and HC for a specific
person (i.e., a person’s household-reported event did not have a matched
counterpart in the person’s corresponding PC records). PCIMPFLG assists analysts
in determining which records have the strongest link to data reported by a
pharmacy. It should be noted that whenever there are multiple purchases of a
unique prescribed medication in a given round, MEPS did not collect information
that would enable designating any single purchase as the “original” purchase at
the time the prescription was first filled, and then designating other purchases
as “refills.” The user needs to keep this in mind when the purchases of a
medication are referred to as “refills” in the documentation. Because matching
was performed at a drug level as opposed to an acquisition level, the values for
PCIMPFLG are either 1 or 2. For more details on general data editing/imputation
methodology, please see Section 4.0.
CLMOMFLG indicates if a prescription medicine event
went through the Charge Payment (CP) section of the HC or was insulin and diabetic supply/equipment (OMTYPE=2 OR 3) that were mentioned in the Other Medical Expenses section of the HC.
Prescription medicine events that went through the CP section of the HC include: (1) events where the person filed their own prescription claim forms with their insurance company,
(2) events for persons for whom the respondent did not know if they filed their
own prescription claim forms with their insurance company, and (3) insulin and
diabetic supply/equipment events (OMTYPE = 2 or 3) that were mentioned in the
Other Medical Expenses section of the HC. Due to design changes, starting with
Panel 17 Round 5 and Panel 18 Round 3, OMTYPE = 2 or 3 events went through the
Charge Payment section only if the family sends in the claim form (PMEDCLM = 1).
For these types of events, information on payment sources was retained to the
extent that these data were reported by the household respondent in the CP
section of the HC.
INPCFLG denotes whether or not a household member had
at least one prescription drug purchase in the PC (0 = NO, 1 = YES).
Return To Table Of Contents
SAMPLE indicates if a respondent reported the person
received a free sample of the prescription medicine in the round (0 = NO, 1 =
YES). Respondents were asked in each round whether or not the person received
any free samples of a reported prescribed medicine during the round. However,
respondents were not asked to report the number of free samples a person
received, nor was it made clear that free samples were included in the count of
the number of times that the respondent reported a person purchasing or
otherwise obtaining the prescribed medicine during the round. It is important
for analysts to note that SAMPLE is not a count variable of free samples;
SAMPLE = 1 indicates that a person was reported getting a free sample of the
prescribed medicine during the round. This flag variable simply allows
individual analysts to determine for themselves how free samples should be
handled in their analysis.
Return To Table Of Contents
Information on household-reported medical conditions
associated with each prescribed medicine event is provided on this file. There
are up to three clinical classification codes listed for each prescribed
medicine event (99.72 percent of prescribed medicine events have 0-3 condition
records linked). To obtain complete information associated with an event, the
analyst must link to the 2013 Medical Conditions File. Details on how to link to
the MEPS 2013 Medical Conditions File are provided in the 2013 Appendix File.
The user should note that, for confidentiality restrictions, provider-reported
condition information (for non-prescription medicines events) is not publicly
available. Provider-reported condition data for non-prescription medicines
events can be accessed only through the MEPS Data Center.
The medical conditions reported by the HC respondent
were recorded by the interviewer as verbatim text, which were then coded to
fully-specified 2013 ICD-9-CM codes, including medical condition, V-codes, and a
small number of E-codes, by professional coders. Although codes were verified
and error rates did not exceed 2 percent for any coder, analysts should not
presume this level of precision in the data; the ability of household
respondents to report condition data that can be coded accurately should not be
assumed. For detailed information on conditions, please refer to the
documentation on the 2013 Medical Conditions File. For frequencies of conditions
by event type, please see the 2013 Appendix File, HC-160I.
The ICD-9-CM condition codes were aggregated into
clinically meaningful categories. These categories, included on the file as
RXCCC1X-RXCCC3X, were generated using Clinical Classification Software (CCS)
(formerly known as Clinical Classifications for Health Care Policy Research
(CCHPR)), which aggregates conditions and V-codes into mutually exclusive
categories, most of which are clinically homogeneous. Starting with the 2013
file, the ICD-9-CM condition and procedure codes variables are omitted.
The clinical classification codes linked to each
prescribed medicine event are sequenced in the order in which the conditions
were reported by the household respondent, which was in chronological order of
reporting and not in order of importance or severity. Analysts who use the 2013
Medical Conditions file in conjunction with this prescribed medicines event file
should note that the conditions on this file are sorted differently than they
appear on the Medical Conditions file.
Return To Table Of Contents
Each record on this file contains the following Multum Lexicon variables:
RXDRGNAM: generic name of the drug most commonly used by prescribing physicians
PREGCAT: pregnancy category variable – identifies the FDA pregnancy category to which a particular drug has been assigned
TCnSn: therapeutic sub-classification variable – assigns one or more sub-categories to a more general therapeutic class category given to a drug
TCnSn_n: therapeutic sub sub-classification variable – assigns one or more sub sub-categories to a more general therapeutic class category and sub-category given to a drug
Beginning with the 2013 data, the variable RXDRGNAM is included on the file.
Users should carefully review the data when conducting
trend analyses or pooling years or panels because Multum’s therapeutic
classification has changed across the years of the MEPS. The Multum variables on
each year of the MEPS Prescribed Medicines files reflect the most recent
classification available in the year the data were released. Since the release
of the 1996 Prescribed Medicines file, the Multum classification has been
changed by the addition of new classes and subclasses, and by changes in the
hierarchy of classes. Three examples follow: 1) In the 1996-2004 Prescribed
Medicines files, antidiabetic drugs are a subclass of the hormone class, but in
subsequent files, the antidiabetic subclass is part of a class of metabolic
drugs. 2) In the 1996-2004 files, antihyperlipidemic agents are categorized as a
class with a number of subclasses including HMG-COA reductase inhibitors (statins).
In subsequent files, antihyperlipidemic drugs are a subclass, and HMG-COA
reductase inhibitors are a sub-subclass, in the metabolic class. 3) In the
1996-2004 files, the psychotherapeutic class comprises drugs from four
subclasses: antidepressants, antipsychotics, anxiolytics/sedatives/hypnotics,
and CNS stimulants. In subsequent files, the psychotherapeutic class comprises
only antidepressants and antipsychotics. Changes may occur between any years.
For additional information on these and other Multum Lexicon variables, as well
as the Multum Lexicon database itself, please refer to
www.multum.com/Lexicon.html.
Users should also be aware of a problem discovered
with the linking between the MEPS Prescribed Medicines files and the Cerner
Multum file that resulted in some incorrect therapeutic classes being assigned.
In particular, some diagnostic tests and medical devices were inadvertently
assigned to be in a therapeutic class when they should not have been.
Specifically, from 1996-2002, some diabetic supplies were assigned to be in
TC1S1 = 101 (sex hormone), and from 2003 through 2010 some diabetic supplies
were assigned to be in TC1S1 = 37 (toxoids). In addition, starting in 2006, NDC
00169750111 should have been assigned to TC1 = 358 and TC1S1 = 99. Analysts
should use caution when using the Cerner Multum therapeutic class variables for
analysis and should always check for accuracy.
Researchers using the Multum Lexicon variables are
requested to cite Multum Lexicon as the data source.
Return To Table Of Contents
Expenditures on this file refer to what is paid for
health care services. More specifically, expenditures in MEPS are defined as the
sum of payments for care received, including out-of-pocket payments and payments
made by private insurance, Medicaid, Medicare, and other sources. The definition
of expenditures used in MEPS differs slightly from its predecessors, the 1987
NMES and 1977 NMCES surveys, where “charges” rather than “sum of payments” were
used to measure expenditures. This change was adopted because charges became a
less appropriate proxy for medical expenditures during the 1990s because of the
increasingly common practice of discounting charges. Although measuring
expenditures as the sum of payments incorporates discounts in the MEPS
expenditure estimates, the estimates do not incorporate any manufacturer or
other rebates associated with Medicaid or other purchases. Another general
change from the two prior surveys is that charges associated with uncollected
liability, bad debt, and charitable care (unless provided by a public clinic or
hospital) are not counted as expenditures, because there are no payments
associated with those classifications. For details on expenditure definitions,
please reference the following, “Informing American Health Care Policy” (Monheit,
Wilson, Arnett, 1999).
If examining trends in MEPS expenditures or performing
longitudinal analysis on MEPS expenditures please refer to Section C,
sub-sections 3.4 and 6.3 respectively for more information.
Return To Table Of Contents
In addition to total expenditures, variables are
provided which itemize expenditures according to major source of payment
categories. These categories are:
- Out-of-pocket by User (self) or Family,
- Medicare,
- Medicaid,
- Private Insurance,
- Veterans Administration/CHAMPVA, excluding TRICARE,
- TRICARE,
- Other Federal Sources – includes Indian Health Service,
military treatment facilities, and other care by the federal
government,
- Other State and Local Source – includes community and
neighborhood clinics, state and local health departments, and
state programs other than Medicaid,
- Workers’ Compensation, and
- Other Unclassified Sources – includes sources such as
automobile, homeowner’s, and liability insurance, and other
miscellaneous or unknown sources.
Two additional source of payment
variables were created to classify payments for events with
apparent inconsistencies between insurance coverage and sources
of payment based on data collected in the survey. These
variables include:
- Other Private – any type of private insurance payments
reported for persons not reported to have any private health
insurance coverage during the year as defined in MEPS, and
- Other Public – Medicare/Medicaid payments reported for
persons who were not reported to be enrolled in the
Medicare/Medicaid program at any time during the year.
Though relatively small in magnitude, data
users/analysts should exercise caution when interpreting the expenditures
associated with these two additional sources of payment. While these payments
stem from apparent inconsistent responses to health insurance and source of
payment questions in the survey, some of these inconsistencies may have logical
explanations. For example, private insurance coverage in MEPS is defined as
having a major medical plan covering hospital and physician services. If a MEPS
sampled person did not have such coverage but had a single service type
insurance plan (e.g., dental insurance) that paid for a particular episode of
care, those payments may be classified as “other private.” Some of the “other
public” payments may stem from confusion between Medicaid and other state and
local programs or may be from persons who were not enrolled in Medicaid, but
were presumed eligible by a provider who ultimately received payments from the
public payer.
Return To Table Of Contents
There is a single full year person-level weight
(PERWT13F) assigned to each record for each key, in-scope person who responded
to MEPS for the full period of time that he or she was
in-scope during 2013. A key person was either a member of a responding NHIS
household at the time of interview or joined a family associated with such a
household after being out-of-scope at the time of the NHIS (the latter
circumstance includes newborns as well as those returning from military service,
an institution, or residence in a foreign country). A person is in-scope
whenever he or she is a member of the civilian noninstitutionalized portion of
the U.S. population.
Return To Table Of Contents
The person-level weight PERWT13F was developed in
several stages. First, person-level weights for Panel 17 and Panel 18 were
created separately. The weighting process for each panel included adjustments
for nonresponse over time and calibration to independent population totals. The
calibration was initially accomplished separately for each panel by raking the
corresponding sample weights for those in-scope at the end of the calendar year
to Current Population Survey (CPS) population estimates based on five variables.
The five variables used in the establishment of the initial person-level control
figures were: census region (Northeast, Midwest, South, West); MSA status (MSA,
non-MSA); race/ethnicity (Hispanic; Black, non-Hispanic; Asian, non-Hispanic;
and other); sex; and age. A 2013 composite weight was then formed by
multiplying each weight from Panel 17 by the factor .51 and each weight from
Panel 18 by the factor .49. The choice of factors reflected the relative sample
sizes of the two panels, helping to limit the variance of estimates obtained
from pooling the two samples. The composite weight was raked to the same set of
CPS-based control totals. When the poverty status information derived from
income variables became available, a final raking was undertaken on the
previously established weight variable. Control totals were established using
poverty status (five categories: below poverty, from 100 to 125 percent of
poverty, from 125 to 200 percent of poverty, from 200 to 400 percent of poverty,
at least 400 percent of poverty), the other five variables previously used in
the weight calibration, and a variable associated with the number of
hospital stays for those under the age of 65.
Return To Table Of Contents
The person-level weight for MEPS Panel 17 was
developed using the 2012 full year weight as a “base” weight for survey
participants present in 2012. For key, in-scope members who joined an RU some
time in 2013 after being out-of-scope in 2012, the initially assigned
person-level weight was the corresponding 2012 family weight. The weighting
process included an adjustment for person-level nonresponse over Rounds 4 and 5
as well as raking to population control totals for December 2013 for key,
responding persons in-scope on December 31, 2013. These control totals were
derived by scaling back the population distribution obtained from the March 2014
CPS to reflect the December 31, 2013 estimated population total (estimated based
on Census projections for January 1, 2014). Variables used for person-level
raking included: census region (Northeast, Midwest, South, West); MSA status
(MSA, non-MSA); race/ethnicity (Hispanic, Black but non-Hispanic; Asian but
non-Hispanic; and other); sex; and age. (Poverty
status is not included in this version of the MEPS full year database because of
the time required to process the income data collected and then assign persons
to a poverty status category). The final weight for key, responding persons who
were not in-scope on December 31, 2013 but were in-scope earlier in the year was
the person weight after the nonresponse adjustment.
Return To Table Of Contents
The person-level weight for MEPS Panel 18 was
developed using the 2013 MEPS Round 1 person-level weight as a “base” weight.
For key, in-scope members who joined an RU after Round 1, the Round 1 family
weight served as a “base” weight. The weighting process included an adjustment
for nonresponse over the remaining data collection rounds in 2013 as well as
raking to the same population control figures for December 2013 used for the
MEPS Panel 17 weights for key, responding persons in-scope on December 31, 2013.
The same five variables employed for Panel 17 raking (census region, MSA status,
race/ethnicity, sex, and age) were used for Panel 18 raking. Again, the final
weight for key, responding persons who were not in-scope on December 31, 2013
but were in-scope earlier in the year was the person weight after the
nonresponse adjustment.
Note that the MEPS Round 1 weights for both panels
incorporated the following components: a weight reflecting the original
household probability of selection for the NHIS and an adjustment for NHIS
nonresponse; a factor representing the proportion of the 16 NHIS panel-quarter
combinations eligible for MEPS; the oversampling of certain subgroups for MEPS
among the NHIS household respondents eligible for MEPS; ratio-adjustment to
NHIS-based national population estimates at the household (occupied DU) level;
adjustment for nonresponse at the DU level for Round 1; and poststratification
to U.S. civilian noninstitutionalized population estimates at the family and
person level obtained from the March CPS databases.
Return To Table Of Contents
year has been described above. In addition, the composite weights of two groups
of persons who were out-of-scope on December 31, 2013 were poststratified.
Specifically, the weights of those who were in-scope some time during the year,
out-of-scope on December 31, and entered a nursing home during the year were
poststratified to a corresponding control total obtained from the 1996 MEPS
Nursing Home Component. The weights of persons who died while in-scope during
2013 were poststratified to corresponding estimates derived using data obtained
from the Medicare Current Beneficiary Survey (MCBS) and Vital Statistics
information provided by the National Center for Health Statistics (NCHS).
Separate decedent control totals were developed for the “65 and older” and “under 65” civilian, noninstitutionalized decedent populations.
In developing the final person-level weight for 2013
(PERWT13F), an additional raking dimension was included to adjust the proportion
of persons under age 65 with at least one inpatient discharge based on
independent sources of data. The table below shows ratios of weighted numbers of
non-elderly persons that resulted from including this additional raking
dimension to that of corresponding estimates without the additional dimension.
Ratio of Adjusted to Unadjusted Weights
| Number of Inpatient Discharges (IPDIS13) |
Non-elderly (AGE13X < 65) |
| 0 |
0.98938 |
| 1+ |
1.21587 |
Overall, the weighted population estimate for the
civilian noninstitutionalized population for December 31, 2013 is 312,098,312
(PERWT13F>0 and INSC1231=1). The sum of the
person-level weights across all persons assigned a positive person-level weight
is 315,721,982.
Return To Table Of Contents
The target population for MEPS in this file is the
2013 U.S. civilian noninstitutionalized population. However, the MEPS sampled
households are a subsample of the NHIS households interviewed in 2011 (Panel 17)
and 2012 (Panel 18). New households created after the NHIS interviews for the
respective panels and consisting exclusively of persons who entered the target
population after 2011 (Panel 17) or after 2012 (Panel 18) are not covered by
MEPS. Neither are previously out-of-scope persons who join an existing household
but are unrelated to the current household residents. Persons not covered by a
given MEPS panel thus include some members of the following groups: immigrants;
persons leaving the military; U.S. citizens returning from residence in another
country; and persons leaving institutions. The set of uncovered persons
constitutes only a small segment of the MEPS target population.
Return To Table Of Contents
MEPS began in 1996, and the utility of the survey for
analyzing health care trends expands with each additional year of data; however,
it is important to consider a variety of factors when examining trends over time
using MEPS. Statistical significance tests should be conducted to assess the
likelihood that observed trends may be attributable to sampling variation. The
adjustment to the weight described in 3.2.3 above based on inpatient discharges
potentially could affect some analyses of trends. The length of time being
analyzed should also be considered. In particular, large shifts in survey
estimates over short periods of time (e.g. from one year to the next) that are
statistically significant should be interpreted with caution, unless they are
attributable to known factors such as changes in public policy, economic
conditions, or MEPS survey methodology.
With respect to methodological considerations,
beginning with the 2007 data, the rules MEPS uses to identify outlier prices for
prescription medications became much less stringent than in prior years.
Starting with the 2007 Prescribed Medicines file, there was: less editing of
prices and quantities reported by pharmacies, more variation in prices for
generics, lower mean prices for
generics, higher mean prices for brand name drugs, greater differences in
prices between generic and brand name drugs, and a somewhat lower proportion of
spending on drugs by families, as opposed to third-party payers. Starting with
the 2008 Prescribed Medicines file, improvements in the data editing changed the
distribution of payments by source: (1) more spending on Medicare beneficiaries
is by private insurance, rather than Medicare, and (2) less out-of-pocket
payments and more Medicaid payments among Medicaid enrollees. Starting with the
2009 data, additional improvements increased public program amounts and reduced
out-of-pocket payments and, for Medicare beneficiaries with both Part D and
Medicaid, decreased Medicare payments and increased Medicaid and other state and
local government payments. Therefore, users should be cautious in the types of
comparisons they make about prescription drug spending before and after 2007,
2008, and 2009. In addition, some therapeutic class codes have changed over
time.
In 2013 MEPS introduced an effort to obtain more
complete information about health care utilization from MEPS respondents with
full implementation in early 2014 at the start of the final rounds of data
collection for 2013. This effort likely resulted in improved data quality and a
reduction in underreporting in 2013, but could have some modest impact on
analyses involving trends in utilization across years.
There are also statistical factors to consider in
interpreting trend analyses. Looking at changes over longer periods of time can
provide a more complete picture of underlying trends. Analysts may wish to
consider techniques to evaluate, smooth, or stabilize analyses of trends such as
comparing pooled time periods (e.g. 1996-97 versus 2012-2013), working with
moving averages, or using modeling techniques with several consecutive years of
MEPS data to test the fit of specified patterns over time. Finally, researchers
should be aware of the impact of multiple comparisons on Type I error. Without
making appropriate allowance for multiple comparisons, undertaking numerous
statistical significance tests of trends increases the likelihood of concluding
that a change has taken place when one has not.
Return To Table Of Contents
The general approach to preparing the household
prescription data for this file was to utilize the PC prescription data to
impute information collected from pharmacy providers to the household drug
mentions. For events that went through the Charge Payment (CP) section of the HC
(events where the person filed their own prescription claim forms with their
insurance company, events for persons for whom the respondent did not know if
they filed their own prescription claim forms with their insurance company, and
insulin and diabetic supply/equipment events (OMTYPE = 2 or 3) that were
mentioned in the Other Medical Expenses section of the HC), information on
payment sources was retained to the extent that these data were reported by the
household respondent in the CP section of the HC. A matching program was adopted
to link PC drugs and the corresponding drug information to household drug
mentions. To improve the quality of these matches, all drugs on the household
and pharmacy files were coded using a proprietary database on the basis of the
medication names provided by the household respondent and pharmacy, and, when
available, the NDC provided in the pharmacy follow-back component. The matching
process was done at a drug (active ingredient) level, as opposed to an
acquisition level. Considerable editing was done prior to the matching to
correct data inconsistencies in both data sets and to fill in missing data and
correct outliers on the pharmacy file.
Drug price-per-unit outliers were analyzed on the
pharmacy file by first identifying the average wholesale unit price (AWUP) of
the drug by linkage through the NDC to a secondary data file. In general,
prescription drug unit prices were deemed to be outliers by comparing unit
prices reported in the pharmacy database to the AWUP reported in the secondary
data file and were edited, as necessary.
Beginning with the 2007 data, the rules used to
identify outlier prices for prescription medications in the PC changed. New
outlier thresholds were established based on the distribution of the ratio of
retail unit prices relative to the AWUP in the 2006 MarketScan Outpatient
Pharmaceutical Claims database. The new thresholds vary by patent status,
whereas in prior years they did not. These changes improve data quality in three
ways: (1) the distribution of prices
in the MEPS better benchmarks to MarketScan, overall and by patent status (Zodet
et al. 2010), (2) fewer pharmacy-reported payments and quantities (for example,
number of pills) are edited, and (3) imputed prices reflect prices paid, rather
than AWUPs. As a result, compared with earlier years of the MEPS, starting with
2007 there is more variation in prices for generics, lower mean prices for
generics, higher mean prices for brand name drugs, greater differences in
prices between generic and brand name drugs, and a somewhat lower proportion of
spending on drugs by families, as opposed to third-party payers. Pharmacy
reports of free antibiotics were not edited as if they were outliers. Beginning
with the 2010 data, some additional free drugs obtained through commercial
pharmacies were not edited.
Beginning with the 2009 data, three changes in editing
sources of payment data were made to improve data quality, based on a validation
study (Hill et al., 2011). Two changes were made in editing fills for which
pharmacies reported partial payment data. First, if the third party amount was
missing and the third party payer was a public payer, then pharmacy reports of
zero out-of-pocket amounts were preserved rather than imputed. Second, somewhat
tighter outlier thresholds were implemented for the fills with partial payment
data, and somewhat looser outlier thresholds were implemented for fills with
complete payment data. Another change affected Medicare beneficiaries with both
Part D and Medicaid coverage--reported Medicaid and other state and local
program payments were no longer edited to be Medicare payments.
Beginning with the 2010 data, improvements in the
payment imputation methods for pharmacy data (1) better utilize
pharmacy-reported quantities to impute missing payment amounts, and
(2) preserve within-NDC variation in the prices on the records for which third
party payment amounts are imputed.
Beginning with the 2011 data, the imputation of the
number of fills for a drug was improved. In the 2011 data, for 10% of
household-reported drugs the respondent did not know or remember the number of
times the drug was obtained during the round. For missing and implausible
values, a hot-deck procedure imputed a new number of acquisitions, drawing from
the donor pool of drugs with valid values. Prior to 2011, the imputation method
gave greater weight to donors with more acquisitions in the round. The new
method conditions on insurance status, age, and geography, as well as drug.
Drug matches between household drug mentions and
pharmacy drug events for a person in the PC were based on drug code, medication
name, and the round in which the drug was reported. The matching of household
drug mentions to pharmacy drugs was performed so that the most detailed and
accurate information for each prescribed medicine event was obtained. Beginning
with the 2008 Prescribed Medicines file, the criteria for matching were changed
to allow multiple NDCs for the same drug reported by pharmacies (for example,
different manufacturers) to match to one drug reported by the household.
Beginning with the 2010 data, the matching process was improved for diabetic
supplies to better utilize pharmacy reports of the diversity of supplies
individuals purchased. Exact dates of purchase were only available from the
follow-back component. The matching program assigned scores to potential
matches. Numeric variables required exact matches to receive a high score, while
partial scores could be assigned to matches between character variables, such as
prescription name, depending on the degree of similarity in the spelling and
sound of the medication names. Household drug mentions that were deemed exact
matches to PC drugs for the same person in the same round required sufficiently
high scores to reflect a high quality match. Initially, exact matches were used
only once and were taken out of the donor pool from that point on (i.e., these
matches were made without replacement). For remaining persons with pharmacy data
from any round and unmatched household drugs, additional matches are made with
replacement across rounds. Any refill of a household drug mention that had been
matched to a pharmacy drug event was matched to the same pharmacy drug event.
All remaining unmatched household drug mentions for persons either in or out of
the PC were statistically matched to the entire pharmacy donor base with
replacement by medication name, drug code, type of third party coverage, health
conditions, age, sex, and other characteristics of the individual. PC records
containing an NDC imputed without an exact match on a generic code were omitted
from the donor pool. Some matches have inconsistencies between the PC donor’s
potential sources of payment and those of the HC recipient, and these were
resolved. Beginning with the 2008 data, the method used to resolve
inconsistencies in potential payers was changed to better reflect the
distribution of sources of payment among the acquisitions with consistent
sources of payment. This change (1) reduced Medicare payments and increased
private payments among Medicare beneficiaries, and
(2) reduced out-of-pocket payments and increased Medicaid payments among
Medicaid enrollees. In addition, Medicare, Medicaid, and private drug
expenditures better benchmark totals in the National Health Expenditure
Accounts.
Also beginning with the 2011 data, many aspects of the
specifications were modified so that imputations and edits better reflect
Medicare Part D donut hole rules and Medicare Part B coverage of a few
medications and diabetic supplies.
For more information on the MEPS Prescribed Medicines
editing and imputation procedures, please see J. Moeller, 2001.
Return To Table Of Contents
Expenditure variables on the 2013 Prescribed Medicines
file have been rounded to the nearest penny. Person-level expenditure variables
released on the 2013 Full Year Consolidated Data File were rounded to the
nearest dollar. It should be noted that using the 2013 MEPS event files to
create person-level totals will yield slightly different totals than those found
on the 2013 Full Year Consolidated data file. These differences are due to
rounding only. Moreover, in some instances, the number of persons having
expenditures on the 2013 event files for a particular source of payment may
differ from the number of persons with expenditures on the 2013 Full Year
Consolidated data file for that source of payment. This difference is also an
artifact of rounding only.
Return To Table Of Contents
There are 13 expenditure variables included on this
event file. All of these expenditures have gone through an editing and
imputation process and have been rounded to the second decimal place. There is a
sum of payments variable (RXXP13X) which, for each prescribed medicine event,
sums all the expenditures from the various sources of payment. The 12 sources of
payment expenditure variables for each prescribed medicine event are the
following: amount paid by self or family (RXSF13X), amount paid by Medicare
(RXMR13X), amount paid by Medicaid (RXMD13X), amount paid by private insurance
(RXPV13X), amount paid by the Veterans Administration/CHAMPVA (RXVA13X), amount
paid by TRICARE (RXTR13X), amount paid by other federal sources (RXOF13X),
amount paid by state and local (non-federal) government sources (RXSL13X),
amount paid by Worker’s Compensation (RXWC13X), and amount paid by some other
source of insurance (RXOT13X). As mentioned previously, there are two additional
expenditure variables called RXOR13X and RXOU13X (other private and other
public, respectively). These two expenditure variables were created to maintain
consistency between what the household respondent reported as a person’s private
and public insurance status for hospitalization and physician coverage and third
party prescription payments from other private and public sources (such as a
separate private prescription policy or prescription coverage from the Veterans
Administration, the Indian Health Service, or a state assistance program other
than Medicaid). Users should exercise caution when interpreting the expenditures
associated with these two additional sources of payment. While these payments
stem from apparent inconsistent responses to health insurance and source of
payment questions in the survey, some of these inconsistencies may have logical
explanations. Please see Section 2.6.4 for details on these and all other source
of payment variables.
Return To Table Of Contents
The data in this file can be used to develop national
2013 event-level estimates for the U.S. civilian noninstitutionalized population
on prescribed medicine purchases (events) as well as expenditures, and sources
of payment for these purchases. Estimates of total number of purchases are the
sum of the weight variable (PERWT13F) across relevant event records while
estimates of other variables must be weighted by PERWT13F to be nationally
representative. The tables below contain event-level estimates for selected
variables.
Selected Event (Purchase) Level Estimates
All Prescribed Medicine Purchases
| Estimate of Interest |
Variable Name |
Estimate (SE) |
| Number of purchases (in millions) |
PERWT13F |
3309.8 (90.04) |
| Mean total payments per purchase |
RXXP13X |
$93 (2.5) |
| Mean out-of-pocket payment per purchase |
RXSF13X |
$15 (0.5) |
| Mean proportion of
expenditures paid by private insurance per purchase |
RXPV13X /RXXP13X |
0.150 (0.0053) |
Example by Drug Type: Statins (TC1S1_1 = 173 or TC1S1_2 = 173 or TC1S2_1 = 173 or TC1S3_1 = 173 or TC2S1_1 = 173 or TC2S1_2 = 173)
| Estimate of Interest |
Variable Name |
Estimate (SE) |
| Number of purchases (in millions) |
PERWT13F |
230.0 (6.86) |
| Mean total payments per purchase |
RXXP13X |
$70 (3.2) |
| Mean annual total payments per person |
RXXP13X (aggregated across purchases within person) |
$393 (17.9) |
Return To Table Of Contents
To enhance analyses of prescribed medicine purchases,
analysts may link information about prescribed medicine purchases to the annual
full year consolidated file (which has data for all MEPS sample persons), or
conversely, link person-level information from the full year consolidated file
to this event-level file (see Section 6 below for more details). Both this file
and the full year consolidated file may be used to derive estimates for persons
with prescribed medicine purchases and annual estimates of total expenditures
for these purchases; however, if the estimate relates to the entire population,
this file cannot be used to calculate the denominator, as only those persons
with at least one prescribed medicine purchase are represented on this data
file. Therefore, the full year consolidated file must be used for person-level
analyses that include both persons with and without prescribed medicine events.
Return To Table Of Contents
It is essential that the analyst examine all variables
for the presence of negative values used to represent missing values. For
continuous or discrete variables, whose means or totals may be calculated, the
analyst should either impute a value or set the value such that it will be
interpreted as missing by the computing language used. For categorical and
dichotomous variables, the analyst may want to consider whether to recode or
impute a value for cases with negative values or whether to exclude or include
such cases in the numerator and/or denominator when calculating proportions.
Methodologies used for the editing/imputation of
expenditure variables (e.g., total expenditures and sources of payment) are
described in Section 4.2.
Return To Table Of Contents
The MEPS is based on a complex sample design. To
obtain estimates of variability (such as the standard error of sample estimates
or corresponding confidence intervals) for MEPS estimates, analysts need to take
into account the complex sample design of MEPS for both person-level and
family-level analyses. Several methodologies have been developed for estimating
standard errors for surveys with a complex sample design, including the
Taylor-series linearization method, balanced repeated replication, and jackknife
replication. Various software packages provide analysts with the capability of
implementing these methodologies. Replicate weights have not been developed for
the MEPS data. Instead, the variables needed to calculate appropriate standard
errors based on the Taylor-series linearization method are included on this file
(as well as all other MEPS public use files). Software packages that permit the
use of the Taylor-series linearization method include SUDAAN, Stata, SAS
(version 8.2 and higher), and SPSS (version 12.0 and higher). For complete
information on the capabilities of each package, analysts should refer to the
corresponding software user documentation.
Using the Taylor-series linearization method, variance
estimation strata and the variance estimation PSUs within these strata must be
specified. The variance strata variable is named VARSTR, while the variance PSU
variable is named VARPSU. Specifying a “with replacement” design in one of the
previously mentioned computer software packages will provide estimated standard
errors appropriate for assessing the variability of MEPS survey estimates. It
should be noted that the number of degrees of freedom associated with estimates
of variability indicated by such a package may not appropriately reflect the
number available. For variables of interest distributed throughout the country
(and thus the MEPS sample PSUs), one can generally expect to have at least 100
degrees of freedom associated with the estimated standard errors for national
estimates based on this MEPS database.
Prior to 2002, MEPS variance strata and PSUs were
developed independently from year to year, and the last two characters of the
strata and PSU variable names denoted the year. However, beginning with the 2002
Point-in-Time PUF, the variance strata and PSUs were developed to be compatible
with MEPS data associated with the NHIS sample design used through 2006. Such
data can be pooled and the variance strata and PSU variables provided can be
used without modification for variance estimation purposes for estimates
covering multiple years of data.
As a result of the change in the NHIS sample design in
2006, a new set of variance strata and PSUs have been established for variance
estimation purposes for use with MEPS Panel 12 and subsequent MEPS panels. There
were 165 variance strata associated with both MEPS Panel 17 and Panel 18,
providing a substantial number of degrees of freedom for subgroups as well as
the nation as a whole. Each variance stratum contains either two or three
variance estimation PSUs.
Return To Table Of Contents
Data from this file can be used alone or in
conjunction with other files for different analytic purposes. This section
summarizes various scenarios for merging/linking MEPS files. Each MEPS panel can
also be linked back to the previous year’s National Health Interview Survey
public use data files. For information on obtaining MEPS/NHIS link files please
see
meps.ahrq.gov/data_stats/more_info_download_data_files.jsp.
Return To Table Of Contents
Merging characteristics of interest from the
person-level file (e.g., MEPS 2013 Full Year Consolidated File) expands the
scope of potential estimates. For example, to estimate the total number of
prescribed medicine purchases of persons with specific demographic
characteristics (such as age, race, sex, and education), population
characteristics from a person-level file need to be merged onto the prescribed
medicines file. This procedure is illustrated below. The MEPS 2013 Appendix
File, HC-160I, provides additional detail on how to merge MEPS data files.
- Create data set PERSX by sorting the 2013 Full Year
Consolidated File by the person identifier, DUPERSID. Keep only
variables to be merged onto the prescribed medicines file and
DUPERSID.
- Create data set PMEDS by sorting the 2013 Prescribed
Medicines File by person identifier, DUPERSID.
- Create final data set NEWPMEDS by merging these two files by
DUPERSID, keeping only records on the prescribed medicines file.
The following is an example of SAS code, which
completes these steps:
PROC SORT DATA=IN.HCXXX (KEEP=DUPERSID AGE31X AGE42X
AGE53X SEX RACEV1X EDRECODE EDUYRDG)
OUT=PERSX;
BY DUPERSID;
RUN;
PROC SORT DATA=IN.HCXXXA
OUT=PMEDS;
BY DUPERSID;
RUN;
DATA NEWPMEDS;
MERGE PMEDS (IN=A) PERSX (IN=B);
BY DUPERSID;
IF A;
RUN;
Return To Table Of Contents
The condition-event link file (CLNK) provides a link
from MEPS event files to the 2013 Medical Conditions File. When using the CLNK,
data users/analysts should keep in mind that
(1) conditions are self-reported, (2) there may be multiple conditions
associated with a prescribed medicine purchase, and (3) a condition may link to
more than one prescribed medicine purchase or any other type of purchase. Users
should also note that not all prescribed medicine purchases link to the
condition file.
Return To Table Of Contents
Panel-specific longitudinal files are available for
downloading in the data section of the MEPS Web site. For each panel, the
longitudinal file comprises MEPS survey data obtained in Rounds 1 through 5 of
the panel and can be used to analyze changes over a two-year period. Variables
in the file pertaining to survey administration, demographics, employment,
health status, disability days, quality of care, patient satisfaction, health
insurance, and medical care use and expenditures were obtained from the MEPS
full-year Consolidated files from the two years covered by that panel.
For more details or to download the data files, please
see Longitudinal Weight Files at
meps.ahrq.gov/data_stats/more_info_download_data_files.jsp.
Return To Table Of Contents
Cohen, S.B. (1998). Sample Design of the 1996 Medical
Expenditure Panel Survey Medical Provider Component. Journal of Economic and
Social Measurement,24, 25-53.
Cohen, S.B. (1996). The Redesign of the Medical
Expenditure Panel Survey: A Component of the DHHS Survey Integration Plan.
Proceedings of the COPAFS Seminar on Statistical Methodology in the Public
Service.
Cox, B.G. and Cohen, S.B. (1985). Imputation
Procedures to Compensate for Missing Responses to Data Items. In D.B. Owen and
R.G.Cornell (Eds.), Methodological Issues for Health Care Surveys (pp.
214-234). New York, NY: Marcel Dekker.
Ezzati-Rice, T.M., Rohde, F., Greenblatt, J. (2008).
Sample Design of the Medical Expenditure Panel Survey Household Component,
1998–2007 (Methodology Report No. 22). Rockville, MD: Agency for Healthcare
Research and Quality.
Hill, S.C., Zuvekas, S.H., and Zodet, M.W. (2011).
Implications of the Accuracy of MEPS Prescription Drug Data for Health Services
Research. Inquiry 48(3). Forthcoming 2011.
Moeller J.F., Stagnitti, M., Horan, E., et al. (2001).
Outpatient Prescription Drugs: Data Collection and
Editing in the 1996 Medical Expenditure Panel Survey (HC-010A) (MEPS
Methodology Report No. 12, AHRQ Pub. No. 01-0002). Rockville, MD: Agency for
Healthcare Research and Quality.
Monheit, A.C., Wilson, R., and Arnett, III, R.H.
(Eds.). (1999) Informing American Health Care Policy. San Francisco, CA:
Jossey-Bass Inc.
Shah, B.V., Barnwell, B.G., Bieler, G.S., Boyle, K.E.,
Folsom, R.E., Lavange, L., Wheeless, S.C., and Williams, R. (1996). Technical
Manual: Statistical Methods and Algorithms Used in SUDAAN Release 7.0.
Research Triangle Park, NC: Research Triangle Institute.
Zodet, M.W., Hill, S.C., and Miller, E. Comparison of
Retail Drug Prices in the MEPS and MarketScan: Implications for MEPS Editing
Rules. Agency for Healthcare Research and Quality Working Paper No. 10001,
February 2010.
Return To Table Of Contents
VARIABLE-SOURCE CROSSWALK
FOR MEPS HC-160A: 2013 Prescribed Medicines Events
Survey Administration Variables
| Variable |
Description |
Source |
| DUID |
Dwelling unit ID |
Assigned in sampling |
| PID |
Person number |
Assigned in sampling |
| DUPERSID |
Sample person ID (DUID + PID) |
Assigned in sampling |
| RXRECIDX |
Record ID – Unique Prescribed Medicine Identifier |
Constructed |
| LINKIDX |
Link to condition and other event files |
CAPI derived |
| DRUGIDX |
Link to drugs across rounds |
CAPI derived |
| PANEL |
Panel indicator |
Assigned in sampling |
| PURCHRD |
Round in which the Rx/prescribed medicine was obtained/purchased |
CAPI derived |
Return To Table Of Contents
Prescribed Medicines Events Variables
| Variable |
Description |
Source |
| RXBEGMM |
Month person first used medicine |
PM12OV1 |
| RXBEGYRX |
Year person first used medicine |
PM12 |
| RXNAME |
Medicine name (Imputed) |
Imputed |
| RXDRGNAM |
Multum medicine name (Imputed) |
Imputed |
| RXNDC |
NDC (Imputed) |
Imputed |
| RXQUANTY |
Quantity of Rx/prescribed medicine (Imputed) |
Imputed |
| RXFORM |
Dosage form (Imputed) |
Imputed |
| RXFRMUNT |
Quantity unit of medication (Imputed) |
Imputed |
| RXSTRENG |
Strength of medication (Imputed) |
Imputed |
| RXSTRUNT |
Unit of medication (Imputed) |
Imputed |
| RXDAYSUP |
Days supplied of prescribed med(Imputed) |
Imputed |
| PHARTP1-PHARTP8 |
Type of pharmacy prov – (1st-8th) |
PM16 |
| RXFLG |
Flag variable indicating imputation source for NDC on pharmacy donor record |
Constructed |
| IMPFLAG |
Method of expenditure data creation |
Constructed |
| PCIMPFLG |
Flag indicating type of household to pharmacy prescription match
|
Constructed |
| CLMOMFLG |
Charge/payment, Rx claim filing, and OMTYPE =2 or =3
(insulin and diabetic supply equipment events) status |
CP01/Constructed |
| INPCFLG |
Flag indicating if the person has at least one record in the pharmacy component |
Constructed |
| SAMPLE |
Flag indicating if a person received a free sample of this drug in the round |
CAPI derived |
| RXCCC1X |
Modified clinical classification code |
Constructed/Edited |
| RXCCC2X |
Modified clinical classification code |
Constructed/Edited
|
| RXCCC3X |
Modified clinical classification code |
Constructed/Edited |
| PREGCAT |
Multum pregnancy category |
Cerner Multum, Inc. |
| TC1 |
Multum therapeutic class #1 |
Cerner Multum, Inc. |
| TC1S1 |
Multum therapeutic sub-class #1 for TC1 |
Cerner Multum, Inc. |
| TC1S1_1 |
Multum therapeutic sub-sub-class for TC1S1 |
Cerner Multum, Inc. |
| TC1S1_2 |
Multum therapeutic sub-sub-class for TC1S1 |
Cerner Multum, Inc. |
| TC1S2 |
Multum therapeutic sub-class #2 for TC1 |
Cerner Multum, Inc. |
| TC1S2_1 |
Multum therapeutic sub-sub-class for TC1S2 |
Cerner Multum, Inc. |
| TC1S3 |
Multum therapeutic sub-class #3 for TC1 |
Cerner Multum, Inc. |
| TC1S3_1 |
Multum therapeutic sub-sub-class for TC1S3 |
Cerner Multum, Inc. |
| TC2 |
Multum therapeutic class #2 |
Cerner Multum, Inc. |
| TC2S1 |
Multum therapeutic sub-class #1 for TC2 |
Cerner Multum, Inc. |
| TC2S1_1 |
Multum therapeutic
sub-sub-class for TC2S1 |
Cerner Multum, Inc. |
| TC2S1_2 |
Multum therapeutic sub-sub-class for TC2S1 |
Cerner Multum, Inc. |
| TC2S2 |
Multum therapeutic sub-class #2 for TC2 |
Cerner Multum, Inc. |
| TC3 |
Multum therapeutic class #3 |
Cerner Multum, Inc. |
| TC3S1 |
Multum therapeutic sub-class #1 for TC3 |
Cerner Multum, Inc. |
| TC3S1_1 |
Multum therapeutic sub-sub-class for TC3S1 |
Cerner Multum, Inc. |
| RXSF13X |
Amount paid, self or family (Imputed) |
CP11/Edited/Imputed |
| RXMR13X |
Amount paid, Medicare (Imputed) |
CP12/CP13/Edited/Imputed |
| RXMD13X |
Amount paid, Medicaid (Imputed) |
CP12/CP13/Edited/Imputed |
| RXPV13X |
Amount paid, private insurance (Imputed) |
CP12/CP13/Edited/Imputed |
| RXVA13X |
Amount paid, Veteran’s Administration/CHAMPVA (Imputed) |
CP12/CP13/Edited/Imputed |
| RXTR13X |
Amount paid, TRICARE (Imputed) |
CP12/CP13/Edited/Imputed |
| RXOF13X |
Amount paid, other Federal (Imputed) |
CP12/CP13/Edited/Imputed |
| RXSL13X |
Amount paid, state and local government (Imputed) |
CP12/CP13/Edited/Imputed |
| RXWC13X |
Amount paid, Worker's Compensation (Imputed) |
CP12/CP13/Edited/Imputed |
| RXOT13X |
Amount paid, other insurance (Imputed) |
CP12/CP13/Edited/Imputed |
| RXOR13X |
Amount paid, other private (Imputed) |
Constructed/Imputed |
| RXOU13X |
Amount paid, other public (Imputed) |
Constructed/Imputed |
| RXXP13X |
Sum of payments RXSF13X – RXOU13X (Imputed) |
CP12/CP13/Edited/Imputed |
Return To Table Of Contents
Weights
| Variable |
Description |
Source |
| PERWT13F |
Final person-level weight |
Constructed |
| VARSTR |
Variance estimation stratum, 2013 |
Constructed |
| VARPSU |
Variance estimation PSU, 2013 |
Constructed |
Return To Table Of Contents
Definitions for RXFORM, Dosage Form
| Dosage Form |
Definition |
| -7 |
refused |
| -8 |
don’t know |
| -9 |
not ascertained |
| ACC |
accessory |
| ACETONIDE |
acetonide |
| ACT |
actuation |
| ADR |
acetic acid drop |
| AE |
aerosol |
| AEPB |
aerosol powder, breath activated |
| AER |
aerosol |
| AER SPRAY |
aerosol spray |
| AERA |
aerosol with adapter |
| AERB |
aerosol, breath activated |
| AERO |
aerosol |
| AEROP |
aerosol powder |
| AEROSOL |
aerosol |
| AERS |
aerosol, solution |
| ALM |
* |
| AMI |
* |
| AMO |
* |
| AMP |
ampule |
| ARA |
aerosol liquid w/adapter (inhaler) |
| ARD |
aerosol solid w/adapter |
| ARO |
aerosol solid |
| ASS |
* |
| AUTO INJ |
auto-injection |
| BACK SUPPORT BELT |
back support belt |
| BAG |
bag |
| BAL |
balm |
| BALM |
balm |
| BAN |
bandage |
| BANDAGE |
bandage |
| BAR |
bar |
| BATTERY |
battery |
| BENCH |
bench |
| BOT |
bottle |
| BOTTLE |
bottle |
| BOX |
box |
| BOXES |
boxes |
| BRACE |
brace |
| BRIEF |
brief |
| BUT |
butterfly |
| C |
capsules, or cream (varies) |
| C12 |
12 hour extended-release capsule |
| C24 |
24 hour extended-release capsule |
| CA |
capsule |
| CANE |
cane |
| CAP |
capsule, caplets |
| CAP DR |
delayed-release capsule |
| CAP ER |
extended-release capsule |
| CAP SA |
slow-acting capsule |
| CAPLET |
caplet |
| CAPLT |
caplet |
| CAPS |
capsules |
| CAPSULE |
capsule |
| CAPSULE SA |
slow-acting capsule |
| CATHETER |
catheter |
| CC |
cubic centimeter |
| CER |
capsule, extended-release tablet, extended-release |
| CHAMBER |
chamber |
| CHEW |
chewable tablet |
| CHEW TAB |
chewable tablet |
| CHEW TABS |
chewable tablets |
| CHEWABLE |
chewable |
| CHW |
chewable tablets |
| CLEANSER |
cleanser |
| COLLAR |
collar |
| COMBO |
* |
| COMPOUND |
compound |
| CON |
condom |
| CONC |
concentrate |
| CONDOM |
condom |
| CONTAINER |
container |
| COS |
* |
| COTTON |
cotton |
| CP12 |
capsule, extended-release, 12 hour |
| CP24 |
capsule, extended-release, 24 hour |
| CPCR |
capsule, extended-release |
| CPDR |
capsule, delayed release |
| CPEP |
capsule, delayed release particles |
| CPSP |
capsule sprinkle |
| CPSR |
slow-release capsule |
| CR |
cream |
| CRE |
cream |
| CREA |
cream |
| CREAM |
cream |
| CRM |
cream |
| CRY |
crystal |
| CRYS |
crystals |
| CRYSTAL |
crystal |
| CTB |
chewable tablets |
| CTG |
cartridge |
| CURVE |
curve |
| CUTTER |
cutter |
| DEV |
device |
| DEVI |
device |
| DEVICE |
device |
| DIA |
diaper |
| DIAPER |
diaper |
| DIAPHRAGM |
diaphragm |
| DIHYDROCHLOR |
dihydrochloride |
| DIPROPION |
dipropionate |
| DIS |
disk, or dermal infusion system |
| DISK |
disk |
| DISKUS |
diskus |
| DISPOSABLE |
disposable |
| DOS PAK |
dose pack |
| DPRH |
diaphragm |
| DR |
drop |
| DRC |
delayed-release capsule |
| DRE |
dressing |
| DRESSING |
dressing |
| DRO |
drop |
| DROP |
drop |
| DROPS |
drops |
| DROPS OPTH OTI |
ophthalmic/otic drops |
| DROPS SUSP |
drops suspension |
| DRP |
drop |
| DRPS |
drops |
| DSK |
disk |
| DSPK |
tablets in a dose pack |
| DSPT |
tablet, dispersible |
| DT |
tablet, disintegrating |
| EAM |
* |
| EAR DROP |
ear drop |
| EAR DROPS |
ear drops |
| EAR DRP |
ear drop |
| EAR SUSP |
ear suspension |
| EC TABS |
enteric coated tablets |
| ECC |
enteric coated capsules |
| ECO |
* |
| ECT |
enteric coated tablets |
| ELI |
elixir |
| ELIX |
elixir |
| ELIXER |
elixir |
| ELIXIR |
elixir |
| ELX |
elixir |
| EMERGENCY KIT |
emergency kit |
| EMO |
emollient |
| EMU |
emulsion |
| EMUL |
emulsion |
| EMULSION |
emulsion |
| ENE |
enema |
| ENEM |
enema |
| ENEMA |
enema |
| ER |
* |
| ERC |
capsule, extended-release |
| ERSUS |
suspension, extended-release |
| ERT |
tablet, extended-release |
| ERTA |
extended-release-tablets |
| ERTC |
tablet, chewable, extended-release |
| ESI |
* |
| EST |
* |
| ETA |
* |
| EXTN CAP |
extended-release capsule |
| EXTRACT |
extract |
| EYE DRO |
eye drop |
| EYE DROP |
eye drop |
| EYE DROPS |
eye drops |
| EYE DRP |
eye drop |
| EYE EMU |
* |
| EYE OIN |
eye ointment |
| EYE SO |
eye solution |
| EYEDRO |
eye drop |
| FIL |
film |
| FILM |
film |
| FILM ER |
film, extended-release |
| FILMTAB |
filmtab |
| FILMTABS |
filmtabs |
| FLOWMETER |
flowmeter |
| FOA |
foam |
| FOAM |
foam |
| GAU |
gauze |
| GAUZE |
gauze |
| GEF |
effervescent granules |
| GEL |
gel |
| GELC |
* |
| GEL CAP |
gel capsule |
| GELS |
gel-forming solution |
| GER |
granule, extended-release |
| GFS |
gel-forming solution |
| GLOVE |
glove |
| GRA |
granules |
| GRAN |
granules |
| GRANULES |
granules |
| GRAR |
granules for reconstitution |
| GRR |
grams |
| GTT |
drops |
| GUL |
* |
| GUM |
gum |
| HFA |
* |
| HOSE |
medical hosiery |
| HU |
capsule |
| HYDROBROMIDE |
hydrobromide |
| ICR |
control-release insert |
| IMPL |
implant |
| IMPLANT |
implant |
| IN |
injectable |
| INH |
inhalant, inhaler |
| INHA |
inhaler |
| INH AER |
inhalant aerosol |
| INHAL |
inhalant |
| INHAL SOL |
inhalant solution |
| INHALER |
inhaler |
| INHL |
inhalant |
| INJ |
injectable |
| INJECTION (S) |
injection (s) |
| INSERT |
insert |
| INST |
insert |
| INSULIN |
insulin |
| IPA |
* |
| IUD |
intrauterine devise |
| IV |
intravenous |
| JEL |
jelly |
| JELLY |
jelly |
| KI |
* |
| KIT |
kit |
| L |
lotion |
| LAN |
* |
| LANCET |
lancet |
| LANCETS |
lancets |
| LI |
liquid |
| LINIMENT |
liniment |
| LIP |
* |
| LIQ |
liquid |
| LIQD |
liquid |
| LIQUID |
liquid |
| LO |
* |
| LOLLIPOP |
lollipop |
| LOT |
lotion |
| LOTION |
lotion |
| LOTN |
lotion |
| LOZ |
lozenge |
| LOZENGE |
lozenge |
| LOZG |
lozenge |
| LPOP |
lollipop |
| LQCR |
liquid, extended-release |
| MALEATE |
maleate |
| MASK |
mask |
| MCG |
microgram |
| MEQ |
milliequivalent |
| METER |
meter |
| MG |
milligram |
| MIS |
miscellaneous |
| MISC |
miscellaneous |
| MIST |
mist |
| MONITOR |
monitor |
| MONOH |
* |
| MOUTHWASH |
mouthwash |
| NAS |
nasal spray |
| NASAL |
nasal |
| NASAL INHALER |
nasal inhaler |
| NASAL POCKET HL |
nasal inhaler, pocket |
| NASAL SOLN |
nasal solution |
| NASAL SPR |
nasal spray |
| NASAL SPRAY |
nasal spray |
| NDL |
needle |
| NE |
nebulizer |
| NEB |
nebulizer |
| NEBU |
nebulization solution |
| NEBULIZER |
nebulizer |
| NEEDLE |
needle |
| NEEDLES |
needles |
| NHL |
* |
| NMA |
enema |
| NMO |
nanomole, millimicromole |
| NOP |
* |
| NOS |
* |
| NOSE DROPS |
nose drops |
| ODR |
ophthalmic drop (ointment) |
| ODT |
oral disintegrating tablet |
| OIL |
oil |
| OIN |
ointment |
| OINT |
ointment |
| OINT TOP |
topical ointment |
| OINTA |
ointment with applicator |
| OINTMENT |
ointment |
| OLN |
* |
| OMB |
* |
| ONT |
ointment |
| OP |
ophthalmic solution |
| OP DROPS |
ophthalmic drops |
| OP SOL |
ophthalmic solution |
| OPA |
* |
| OPH |
ophthalmic |
| OPH S |
ophthalmic solution or suspension |
| OPH SOL |
ophthalmic solution |
| OPH SOLN |
ophthalmic solution |
| OPHT SOL |
ophthalmic solution |
| OPHTH DROP (S) |
ophthalmic drops |
| OPHTH OINT |
ophthalmic ointment |
| OPHTH SOLN |
ophthalmic solution |
| OPT SLN |
ophthalmic solution |
| OPT SOL |
ophthalmic solution |
| OPTH |
ophthalmic solution or suspension or ointment |
| OPTH S |
ophthalmic solution or suspension |
| OPTH SLN |
ophthalmic solution |
| OPTH SOL |
ophthalmic solution |
| OPTH SUSP |
ophthalmic suspension |
| OPTIC |
optic |
| ORA |
* |
| ORAL |
oral |
| ORAL INHL |
oral inhalant |
| ORAL INHALER |
oral inhaler |
| ORAL PWD |
oral powder |
| ORAL RINSE |
oral rinse |
| ORAL SOL |
oral solution |
| ORAL SUS |
oral suspension |
| ORAL SUSP |
oral suspension |
| ORM |
* |
| OSE |
* |
| OTHER |
other |
| OTI |
otic solution |
| OTIC |
otic |
| OTIC SOL |
otic solution |
| OTIC SOLN |
otic solution |
| OTIC SUS |
otic suspension |
| OTIC SUSP |
otic suspension |
| PA |
tablet pack, pad or patch (varies) |
| PAC |
pack |
| PACK |
pack |
| PAD |
pad |
| PADS |
pads |
| PAK |
pack |
| PAS |
paste |
| PASTE |
paste |
| PAT |
patch |
| PATCH |
patch |
| PATCHES |
patches |
| PCH |
patch |
| PDI |
powder for injection |
| PDR |
powder |
| PDS |
powder for reconstitution |
| PEDIATRIC DROPS |
pediatric drops |
| PEL |
pellets |
| PEN |
pen |
| PI1 |
powder for injection, 1 month |
| PI3 |
powder for injection, 3 months |
| PIH |
powder for inhalation |
| PKG |
package |
| PKT |
packet |
| PLASTER |
plaster |
| PLEDGETS |
pledgets |
| PLLT |
pellet |
| PO-SYRUP |
syrup by mouth (oral syrup) |
| POD |
POD |
| POPSICLE |
popsicle |
| POUCH |
pouch |
| POW |
powder |
| POWD |
powder |
| POWDER |
powder |
| POWDER/SUSPENS |
powder/suspension |
| PRO |
prophylactic |
| PST |
paste |
| PSTE |
paste |
| PT24 |
patch, 24 hour |
| PT72 |
patch, 72 hour |
| PTCH |
patch |
| PTTW |
patch, biweekly |
| PTWK |
patch, weekly |
| PULVULE |
pulvule |
| PWD |
powder |
| PWD F/SOL |
powder for solution |
| PWDI |
powder for injection |
| PWDIE |
powder for injection, extended-release |
| PWDR |
powder for reconstitution |
| PWDRD |
powder for reconstitution, delayed-release |
| RAL |
* |
| RCTL SUPP |
rectal suppository |
| RECTAL CREAM |
rectal cream |
| REDITABS |
reditabs |
| REF |
* |
| RIN |
rinse |
| RING |
ring |
| RINSE |
rinse |
| RMO |
* |
| ROLL |
roll |
| RTL |
* |
| S |
syrup, suspension, solution (varies) |
| SA CAPS |
slow-acting capsules |
| SA TAB |
slow-acting tablet |
| SA TABLETS |
slow-acting tablets |
| SA TABS |
slow-acting tablets |
| SAL |
salve |
| SALIC |
* |
| SCRUB |
scrub |
| SE |
* |
| SER |
extended-release suspension |
| SET |
set |
| SGL |
soft b23gel cap |
| SHA |
shampoo |
| SHAM |
shampoo |
| SHAMPOO |
shampoo |
| SHMP |
shampoo |
| SHOE |
shoe |
| SLT |
sublingual tablet |
| SL TAB |
sublingual tablet |
| SO |
solution |
| SOA |
soap |
| SOL |
solution |
| SOLG |
gel forming solution |
| SOLN |
solution |
| SOLR |
solution, reconstituted |
| SOLUTION |
solution |
| SOLU |
solution |
| SP |
spray |
| SPG |
sponge |
| SPN |
* |
| SPONGE |
sponge |
| SPR |
spray |
| SPRAY |
spray |
| SQU |
* |
| SRN |
syringe |
| ST |
* |
| STA |
* |
| STAT |
immediately |
| STK |
stick |
| STOCKING |
stocking |
| STP |
strip |
| STR |
strip |
| STRIP |
strip |
| STRIPS |
strips |
| STRP |
strip |
| SU |
suspension, solution, suppository, powder, or granules for
reconstitution (varies) |
| SUB |
sublingual |
| SUBL |
tablet, sublingual |
| SUBLINGUAL |
sublingual |
| SUP |
suppository |
| SUPP |
suppository |
| SUPPOSITORIES |
suppositories |
| SUPPOSITORY |
suppository |
| SUS |
suspension |
| SUS/LIQ |
suspension/liquid |
| SUSP |
suspension |
| SUSPEN |
suspension |
| SUSPENDED RELEASE CAPLET |
suspended release caplet |
| SUSPENSION |
suspension |
| SUSR |
suspension, reconstituted |
| SWA |
swab |
| SWAB |
swab |
| SWABS |
swabs |
| SYG |
* |
| SYP |
syrup |
| SYR |
syrup |
| SYRG |
syringe |
| SYRINGE |
syringe |
| SYRP |
syrup |
| SYRUP |
syrup |
| T |
tablet |
| T12 |
12 hour extended-release tablet |
| T12A |
12 hour extended-release tablet |
| T24 |
24 hour extended-release tablet |
| T24A |
24 hour extended-release tablet |
| TA |
tablet |
| TAB |
tablet |
| TAB CHEW |
chewable tablet |
| TAB DR |
delayed-release tablet |
| TAB EC |
enteric coated tablet |
| TAB SL |
slow-acting tablet |
| TAB SUBL |
sublingual tablet |
| TABL |
tablet |
| TABLET |
tablet |
| TABLET CUTTER |
tablet cutter |
| TABLET SPLITTER |
tablet splitter |
| TABLETS |
tablets |
| TABS |
tablets |
| TAM |
tampon |
| TAP |
tape |
| TAPE |
tape |
| TB |
tablet |
| TB12 |
tablet, extended-release 12 hour |
| TB24 |
tablet, extended-release 24 hour |
| TBCH |
chewable tablet |
| TBCR |
tablet, extended-release |
| TBDP |
tablet, dispersible |
| TBEC |
tablet, delayed-release |
| TBEF |
tablet effervescent |
| TBS |
tablets |
| TBSL |
sublingual tablet |
| TBSO |
tablet, soluble |
| TBSR |
slow-release tablet |
| TC |
tablet, chewable |
| TCP |
tablet, coated particles |
| TDM |
extended-release film |
| TDR |
orally disintegrating tablets |
| TDS |
transdermal system |
| TEF |
effervescent tablet |
| TER |
extended-release tablet |
| TERF |
film, extended-release |
| TES |
test |
| TEST |
test |
| TEST STRIP |
test strip |
| TEST STRIPS |
test strips |
| TIN |
tincture |
| TINC |
tincture |
| TOP CREAM |
topical cream |
| TOP OINT |
topical ointment |
| TOP SOL |
topical solution |
| TOP SOLN |
topical solution |
| TOPICAL |
topical |
| TOPICAL CREAM |
topical cream |
| TOPICAL GEL |
topical gel |
| TOPICAL OINTMENT |
topical ointment |
| TOPICAL SOLUTION |
topical solution |
| TRO |
troche |
| TROC |
troche |
| TROCHE |
troche |
| TTB |
time release tablet |
| TUB |
tube |
| TUBE |
tube |
| UNDERWEAR |
underwear |
| UNIT DOSE |
unit dose |
| UNT |
unit |
| VAGINAL CREAM |
vaginal cream |
| VAPORIZER |
vaporizer |
| VIA |
vial |
| VIAL |
vial |
| VIAL(S) |
vial(s) |
| VIL |
vial |
| WAB |
* |
| WAF |
wafer |
| WAFR |
wafer |
| WALKER |
walker |
| WASH |
wash |
| WIPES |
wipes |
| Z-PAK |
z-pak |
* No definition for the dosage form.
Return To Table Of Contents
Definitions for RXFRMUNT, Quantity Unit of Medication
| Code |
Description |
| -1 |
inapplicable |
| -7 |
refused |
| -8 |
don’t know |
| -9 |
not ascertained |
| ALCOHOL PADS |
alcohol pads |
| CAPLT |
caplet |
| CAPS |
capsule |
| CC |
cubic centimeter |
| EA |
each |
| G |
gram |
| GELC |
* |
| GM |
gram |
| GR |
gram |
| INH |
inhaler |
| L |
liter |
| LANCETS |
lancets |
| LOZ |
lozenge |
| MCL |
microliter |
| MCM |
micrometer |
| MCN |
* |
| MG |
milligram |
| ML |
milliliter |
| MONITOR |
monitor |
| NDL |
* |
| OTHER |
other |
| PA |
* |
| PT |
* |
| SRN |
* |
| SUP |
* |
| TEST STRIPS |
test strips |
| OZ |
ounce |
| QT |
quart |
| TAB |
tablet |
* No description for the code.
Return To Table Of Contents
Definitions for RXSTRUNT, Unit of Medication
| Abbreviations, Codes and Symbols |
Definition |
| -7 |
refused |
| -8 |
don't know |
| -9 |
not ascertained |
| % |
percent |
| 09 |
compound |
| 9HR |
9hr |
| 24HR |
24hr |
| 91 |
other specify |
| ACT |
actuation |
| ACTIVATION |
activation |
| ACTUATION |
actuation |
| BLIST |
blister |
| B CELL |
b cell |
| CC |
cubic centimeters |
| CM2 |
square centimeter |
| DOSE |
dose |
| DROP |
drop |
| DRP |
drop |
| EL |
ELISA (enzyme linked immunosorbent assay) |
| G |
gram |
| GM |
gram |
| GR |
grain |
| HR or HRS |
hour, hours |
| INH |
inhalation |
| IU |
international unit |
| MCG |
microgram |
| MEQ |
milliequivalent |
| MG |
milligram |
| ML |
milliliter |
| MM |
millimeter |
| MMU |
millimass units |
| MU |
* |
| OTHER |
other |
| OZ |
ounce |
| PACKET |
packet |
| PFU |
plaque forming units |
| SPRAY |
spray |
| SQ CM |
square centimeter |
| U or UNIT |
units |
| UNT |
unit |
| VIAL |
vial |
* No definition for the abbreviations, codes and symbols.
Return To Table Of Contents
Definitions of Therapeutic Class Code
| Therapeutic Class Code |
Definition |
| -9 |
not ascertained |
| -1 |
inapplicable |
| 1 |
anti-infectives |
| 2 |
amebicides |
| 3 |
anthelmintics |
| 4 |
antifungals |
| 5 |
antimalarial agents |
| 6 |
antituberculosis agents |
| 7 |
antiviral agents |
| 8 |
carbapenems |
| 9 |
cephalosporins |
| 10 |
leprostatics |
| 11 |
macrolide derivatives |
| 12 |
miscellaneous antibiotics |
| 13 |
penicillins |
| 14 |
quinolones |
| 15 |
sulfonamides |
| 16 |
tetracyclines |
| 17 |
urinary anti-infectives |
| 18 |
aminoglycosides |
| 19 |
antihyperlipidemic agents |
| 20 |
antineoplastics |
| 21 |
alkylating agents |
| 22 |
antineoplastic antibiotics |
| 23 |
antimetabolites |
| 24 |
antineoplastic hormones |
| 25 |
miscellaneous antineoplastics |
| 26 |
mitotic inhibitors |
| 27 |
radiopharmaceuticals |
| 28 |
biologicals |
| 30 |
antitoxins and antivenins |
| 31 |
bacterial vaccines |
| 32 |
colony stimulating factors |
| 33 |
immune globulins |
| 34 |
in vivo diagnostic biologicals |
| 36 |
recombinant human erythropoietins |
| 37 |
toxoids |
| 38 |
viral vaccines |
| 39 |
miscellaneous biologicals |
| 40 |
cardiovascular agents |
| 41 |
agents for hypertensive emergencies |
| 42 |
angiotensin converting enzyme inhibitors |
| 43 |
antiadrenergic agents, peripherally acting |
| 44 |
antiadrenergic agents, centrally acting |
| 45 |
antianginal agents |
| 46 |
antiarrhythmic agents |
| 47 |
beta-adrenergic blocking agents |
| 48 |
calcium channel blocking agents |
| 49 |
diuretics |
| 50 |
inotropic agents |
| 51 |
miscellaneous cardiovascular agents |
| 52 |
peripheral vasodilators |
| 53 |
vasodilators |
| 54 |
vasopressors |
| 55 |
antihypertensive combinations |
| 56 |
angiotensin II inhibitors |
| 57 |
central nervous system agents |
| 58 |
analgesics |
| 59 |
miscellaneous analgesics |
| 60 |
narcotic analgesics |
| 61 |
nonsteroidal anti-inflammatory agents |
| 62 |
salicylates |
| 63 |
analgesic combinations |
| 64 |
anticonvulsants |
| 65 |
antiemetic/antivertigo agents |
| 66 |
antiparkinson agents |
| 67 |
anxiolytics, sedatives, and hypnotics |
| 68 |
barbiturates |
| 69 |
benzodiazepines |
| 70 |
miscellaneous anxiolytics, sedatives and hypnotics |
| 71 |
CNS stimulants |
| 72 |
general anesthetics |
| 73 |
muscle relaxants |
| 74 |
neuromuscular blocking agents |
| 76 |
miscellaneous antidepressants |
| 77 |
miscellaneous antipsychotic agents |
| 79 |
psychotherapeutic combinations |
| 80 |
miscellaneous central nervous system agents |
| 81 |
coagulation modifiers |
| 82 |
anticoagulants |
| 83 |
antiplatelet agents |
| 84 |
heparin antagonists |
| 85 |
miscellaneous coagulation modifiers |
| 86 |
thrombolytics |
| 87 |
gastrointestinal agents |
| 88 |
antacids |
| 89 |
anticholinergics/antispasmodics |
| 90 |
antidiarrheals |
| 91 |
digestive enzymes |
| 92 |
gallstone solubilizing agents |
| 93 |
GI stimulants |
| 94 |
H2 antagonists |
| 95 |
laxatives |
| 96 |
miscellaneous GI
agents |
| 97 |
hormones/hormone modifiers |
| 98 |
adrenal cortical steroids |
| 99 |
antidiabetic agents |
| 100 |
miscellaneous hormones |
| 101 |
sex hormones |
| 102 |
contraceptives |
| 103 |
thyroid hormones |
| 104 |
immunosuppressive
agents |
| 105 |
miscellaneous agents |
| 106 |
antidotes |
| 107 |
chelating agents |
| 108 |
cholinergic muscle stimulants |
| 109 |
local injectable anesthetics |
| 110 |
miscellaneous uncategorized agents |
| 111 |
psoralens |
| 112 |
radiocontrast agents |
| 113 |
genitourinary tract agents |
| 114 |
illicit (street) drugs |
| 115 |
nutritional products |
| 116 |
iron products |
| 117 |
minerals and electrolytes |
| 118 |
oral nutritional supplements |
| 119 |
vitamins |
| 120 |
vitamin and mineral combinations |
| 121 |
intravenous nutritional products |
| 122 |
respiratory agents |
| 123 |
antihistamines |
| 124 |
antitussives |
| 125 |
bronchodilators |
| 126 |
methylxanthines |
| 127 |
decongestants |
| 128 |
expectorants |
| 129 |
miscellaneous
respiratory agents |
| 130 |
respiratory inhalant products |
| 131 |
antiasthmatic combinations |
| 132 |
upper respiratory combinations |
| 133 |
topical agents |
| 134 |
anorectal preparations |
| 135 |
antiseptic and germicides |
| 136 |
dermatological agents |
| 137 |
topical anti-infectives |
| 138 |
topical steroids |
| 139 |
topical anesthetics |
| 140 |
miscellaneous topical agents |
| 141 |
topical steroids with anti-infectives |
| 143 |
topical acne agents |
| 144 |
topical antipsoriatics |
| 146 |
mouth and throat products |
| 147 |
ophthalmic preparations |
| 148 |
otic preparations |
| 149 |
spermicides |
| 150 |
sterile irrigating
solutions |
| 151 |
vaginal preparations |
| 153 |
plasma expanders |
| 154 |
loop diuretics |
| 155 |
potassium-sparing
diuretics |
| 156 |
thiazide diuretics |
| 157 |
carbonic anhydrase inhibitors |
| 158 |
miscellaneous diuretics |
| 159 |
first generation cephalosporins |
| 160 |
second generation cephalosporins |
| 161 |
third generation cephalosporins |
| 162 |
fourth generation cephalosporins |
| 163 |
ophthalmic anti-infectives |
| 164 |
ophthalmic glaucoma agents |
| 165 |
ophthalmic steroids |
| 166 |
ophthalmic steroids with anti-infectives |
| 167 |
ophthalmic anti-inflammatory agents |
| 168 |
ophthalmic lubricants and irrigations |
| 169 |
miscellaneous
ophthalmic agents |
| 170 |
otic anti-infectives |
| 171 |
otic steroids with
anti-infectives |
| 172 |
miscellaneous otic
agents |
| 173 |
HMG-CoA reductase inhibitors |
| 174 |
miscellaneous antihyperlipidemic agents |
| 175 |
protease inhibitors |
| 176 |
NRTIs |
| 177 |
miscellaneous antivirals |
| 178 |
skeletal muscle relaxants |
| 179 |
skeletal muscle relaxant combinations |
| 180 |
adrenergic bronchodilators |
| 181 |
bronchodilator combinations |
| 182 |
androgens and anabolic steroids |
| 183 |
estrogens |
| 184 |
gonadotropins |
| 185 |
progestins |
| 186 |
sex hormone combinations |
| 187 |
miscellaneous sex hormones |
| 191 |
narcotic analgesic
combinations |
| 192 |
antirheumatics |
| 193 |
antimigraine agents |
| 194 |
antigout agents |
| 195 |
5HT3 receptor antagonists |
| 196 |
phenothiazine antiemetics |
| 197 |
anticholinergic antiemetics |
| 198 |
miscellaneous antiemetics |
| 199 |
hydantoin anticonvulsants |
| 200 |
succinimide anticonvulsants |
| 201 |
barbiturate anticonvulsants |
| 202 |
oxazolidinedione anticonvulsants |
| 203 |
benzodiazepine anticonvulsants |
| 204 |
miscellaneous anticonvulsants |
| 205 |
anticholinergic antiparkinson agents |
| 206 |
miscellaneous antiparkinson agents |
| 208 |
SSRI antidepressants |
| 209 |
tricyclic antidepressants |
| 210 |
phenothiazine antipsychotics |
| 211 |
platelet aggregation inhibitors |
| 212 |
glycoprotein platelet inhibitors |
| 213 |
sulfonylureas |
| 214 |
biguanides |
| 215 |
insulin |
| 216 |
alpha-glucosidase inhibitors |
| 217 |
bisphosphonates |
| 218 |
alternative medicines |
| 219 |
nutraceutical products |
| 220 |
herbal products |
| 222 |
penicillinase resistant penicillins |
| 223 |
antipseudomonal penicillins |
| 224 |
aminopenicillins |
| 225 |
beta-lactamase inhibitors |
| 226 |
natural penicillins |
| 227 |
NNRTIs |
| 228 |
adamantane antivirals |
| 229 |
purine nucleosides |
| 230 |
aminosalicylates |
| 231 |
nicotinic acid derivatives |
| 232 |
rifamycin derivatives |
| 233 |
streptomyces derivatives |
| 234 |
miscellaneous
antituberculosis agents |
| 235 |
polyenes |
| 236 |
azole antifungals |
| 237 |
miscellaneous antifungals |
| 238 |
antimalarial quinolines |
| 239 |
miscellaneous antimalarials |
| 240 |
lincomycin derivatives |
| 241 |
fibric acid derivatives |
| 242 |
psychotherapeutic
agents |
| 243 |
leukotriene modifiers |
| 244 |
nasal lubricants and
irrigations |
| 245 |
nasal steroids |
| 246 |
nasal antihistamines and decongestants |
| 247 |
nasal preparations |
| 248 |
topical emollients |
| 249 |
antidepressants |
| 250 |
monoamine oxidase inhibitors |
| 251 |
antipsychotics |
| 252 |
bile acid sequestrants |
| 253 |
anorexiants |
| 254 |
immunologic agents |
| 256 |
interferons |
| 257 |
immunosuppressive monoclonal antibodies |
| 261 |
heparins |
| 262 |
coumarins and indandiones |
| 263 |
impotence agents |
| 264 |
urinary antispasmodics |
| 265 |
urinary pH modifiers |
| 266 |
miscellaneous genitourinary tract agents |
| 267 |
ophthalmic antihistamines and decongestants |
| 268 |
vaginal anti-infectives |
| 269 |
miscellaneous vaginal agents |
| 270 |
antipsoriatics |
| 271 |
thiazolidinediones |
| 272 |
proton pump inhibitors |
| 273 |
lung surfactants |
| 274 |
cardioselective beta blockers |
| 275 |
non-cardioselective beta blockers |
| 276 |
dopaminergic antiparkinsonism agents |
| 277 |
5-aminosalicylates |
| 278 |
cox-2 inhibitors |
| 279 |
gonadotropin-releasing hormone and analogs |
| 280 |
thioxanthenes |
| 281 |
neuraminidase inhibitors |
| 282 |
meglitinides |
| 283 |
thrombin inhibitors |
| 284 |
viscosupplementation agents |
| 285 |
factor Xa inhibitors |
| 286 |
mydriatics |
| 287 |
ophthalmic anesthetics |
| 288 |
5-alpha-reductase inhibitors |
| 289 |
antihyperuricemic agents |
| 290 |
topical antibiotics |
| 291 |
topical antivirals |
| 292 |
topical antifungals |
| 293 |
glucose elevating agents |
| 295 |
growth hormones |
| 296 |
inhaled corticosteroids |
| 297 |
mucolytics |
| 298 |
mast cell stabilizers |
| 299 |
anticholinergic bronchodilators |
| 300 |
corticotropin |
| 301 |
glucocorticoids |
| 302 |
mineralocorticoids |
| 303 |
agents for pulmonary hypertension |
| 304 |
macrolides |
| 305 |
ketolides |
| 306 |
phenylpiperazine antidepressants |
| 307 |
tetracyclic antidepressants |
| 308 |
SSNRI antidepressants |
| 309 |
miscellaneous antidiabetic agents |
| 310 |
echinocandins |
| 311 |
dibenzazepine anticonvulsants |
| 312 |
cholinergic agonists |
| 313 |
cholinesterase inhibitors |
| 314 |
antidiabetic combinations |
| 315 |
glycylcyclines |
| 316 |
cholesterol absorption inhibitors |
| 317 |
antihyperlipidemic combinations |
| 318 |
insulin-like growth factor |
| 319 |
vasopressin antagonists |
| 320 |
smoking cessation agents |
| 321 |
ophthalmic diagnostic agents |
| 322 |
ophthalmic surgical agents |
| 323 |
antineoplastic monoclonal antibodies |
| 324 |
antineoplastic interferons |
| 325 |
sclerosing agents |
| 327 |
antiviral combinations |
| 328 |
antimalarial combinations |
| 329 |
antituberculosis combinations |
| 330 |
antiviral interferons |
| 331 |
radiologic agents |
| 332 |
radiologic adjuncts |
| 333 |
miscellaneous iodinated contrast media |
| 334 |
lymphatic staining agents |
| 335 |
magnetic resonance imaging contrast media |
| 336 |
non-iodinated contrast media |
| 337 |
ultrasound contrast media |
| 338 |
diagnostic radiopharmaceuticals |
| 339 |
therapeutic radiopharmaceuticals |
| 340 |
aldosterone receptor antagonists |
| 341 |
atypical antipsychotics |
| 342 |
renin inhibitors |
| 343 |
tyrosine kinase inhibitors |
| 344 |
nasal anti-infectives |
| 345 |
fatty acid derivative anticonvulsants |
| 346 |
gamma-aminobutyric acid reuptake inhibitors |
| 347 |
gamma-aminobutyric acid analogs |
| 348 |
triazine anticonvulsants |
| 349 |
carbamate anticonvulsants |
| 350 |
pyrrolidine anticonvulsants |
| 351 |
carbonic anhydrase inhibitor anticonvulsants |
| 352 |
urea anticonvulsants |
| 353 |
anti-angiogenic ophthalmic agents |
| 354 |
H. pylori eradication agents |
| 355 |
functional bowel disorder agents |
| 356 |
serotoninergic neuroenteric modulators |
| 357 |
growth hormone receptor blockers |
| 358 |
metabolic agents |
| 359 |
peripherally acting antiobesity agents |
| 360 |
lysosomal enzymes |
| 361 |
miscellaneous metabolic agents |
| 362 |
chloride channel activators |
| 363 |
probiotics |
| 364 |
antiviral chemokine receptor antagonist |
| 365 |
medical gas |
| 366 |
integrase strand transfer inhibitor |
| 368 |
non-ionic iodinated contrast media |
| 369 |
ionic iodinated contrast media |
| 370 |
otic steroids |
| 371 |
dipeptidyl peptidase 4 inhibitors |
| 372 |
amylin analogs |
| 373 |
incretin mimetics |
| 374 |
cardiac stressing agents |
| 375 |
peripheral opioid receptor antagonists |
| 376 |
radiologic conjugating agents |
| 377 |
prolactin inhibitors |
| 378 |
drugs used in alcohol dependence |
| 379 |
next generation cephalosporins |
| 380 |
topical debriding agents |
| 381 |
topical depigmenting agents |
| 382 |
topical antihistamines |
| 383 |
antineoplastic detoxifying agents |
| 384 |
platelet-stimulating agents |
| 385 |
group I antiarrhythmics |
| 386 |
group II antiarrhythmics |
| 387 |
group III antiarrhythmics |
| 388 |
group IV antiarrhythmics |
| 389 |
group V antiarrhythmics |
| 390 |
hematopoietic stem cell mobilizer |
| 391 |
mTOR kinase inhibitors |
| 392 |
otic anesthetics |
| 393 |
cerumenolytics |
| 394 |
topical astringents |
| 395 |
topical keratolytics |
| 396 |
prostaglandin D2 antagonists |
| 397 |
multikinase inhibitors |
| 398 |
BCR-ABL tyrosine kinase inhibitors |
| 399 |
CD52 monoclonal antibodies |
| 400 |
CD33 monoclonal antibodies |
| 401 |
CD20 monoclonal antibodies |
| 402 |
VEGF/VEGFR inhibitors |
| 403 |
mTOR inhibitors |
| 404 |
EGFR inhibitors |
| 405 |
HER2 inhibitors |
| 406 |
glycopeptide antibiotics |
| 407 |
inhaled anti-infectives |
| 408 |
histone deacetylase inhibitors |
| 409 |
bone resorption inhibitors |
| 410 |
adrenal corticosteroid inhibitors |
| 411 |
calcitonin |
| 412 |
uterotonic agents |
| 413 |
antigonadotropic agents |
| 414 |
antidiuretic hormones |
| 415 |
miscellaneous bone resorption inhibitors |
| 416 |
somatostatin and somatostatin analogs |
| 417 |
selective estrogen receptor modulators |
| 418 |
parathyroid hormone and analogs |
| 419 |
gonadotropin-releasing hormone antagonists |
| 420 |
antiandrogens |
| 422 |
antithyroid agents |
| 423 |
aromatase inhibitors |
| 424 |
estrogen receptor antagonists |
| 426 |
synthetic ovulation stimulants |
| 427 |
tocolytic agents |
| 428 |
progesterone receptor modulators |
| 429 |
trifunctional monoclonal antibodies |
| 430 |
anticholinergic chronotropic agents |
| 431 |
anti-CTLA-4 monoclonal antibodies |
| 432 |
vaccine combinations |
| 433 |
Catecholamines |
| 435 |
selective phosphodiesterase-4 inhibitors |
| 437 |
Immunostimulants |
| 438 |
Interleukins |
| 439 |
other immunostimulants |
| 440 |
therapeutic vaccines |
| 441 |
calcineurin inhibitors |
| 442 |
TNF alfa inhibitors |
| 443 |
interleukin inhibitors |
| 444 |
selective immunosuppressants |
| 445 |
other immunosuppressants |
| 446 |
neuronal potassium channel openers |
| 447 |
CD30 monoclonal antibodies |
| 448 |
topical non-steroidal anti-inflammatories |
| 449 |
hedgehog pathway inhibitors |
| 450 |
topical antineoplastics |
| 451 |
topical photochemotherapeutics |
| 452 |
CFTR potentiators |
| 453 |
topical rubefacient |
| 454 |
proteasome inhibitors |
| 455 |
guanylate cyclase-c agonists |
| 456 |
ampa receptor antagonists |
| 457 |
hydrazide derivatives |
| 458 |
sglt-2 inhibitors |
| 459 |
urea cycle disorder agents |
| 460 |
phosphate binders |
| 461 |
topical anti-rosacea agents |
| 462 |
allergenics |
| 463 |
protease-activated receptor-1 antagonists |
| 464 |
miscellaneous diagnostic dyes |
| 465 |
diarylquinolines
|
| 466 |
bone morphogenetic proteins |
| 467 |
ace inhibitors with thiazides |
| 468 |
antiadrenergic agents (central) with thiazides |
| 469 |
antiadrenergic agents (peripheral) with thiazides |
| 470 |
miscellaneous antihypertensive combinations |
| 472 |
beta blockers with thiazides |
| 473 |
angiotensin II inhibitors with thiazides |
| 474 |
beta blockers with calcium channel blockers |
| 475 |
potassium sparing diuretics with thiazides |
| 476 |
ace inhibitors with calcium channel blocking agents |
| 479 |
angiotensin II inhibitors with calcium channel blockers |
Return To Table Of Contents
|
