
|
|
Font Size:
|
||||
|
|
|
|
||||
STATISTICAL BRIEF #400:
Trends in Urinary Antispasmodics Utilization and Expenditures for the U.S. Civilian Noninstitutionalized Population, 2000 and 2010
Highlights
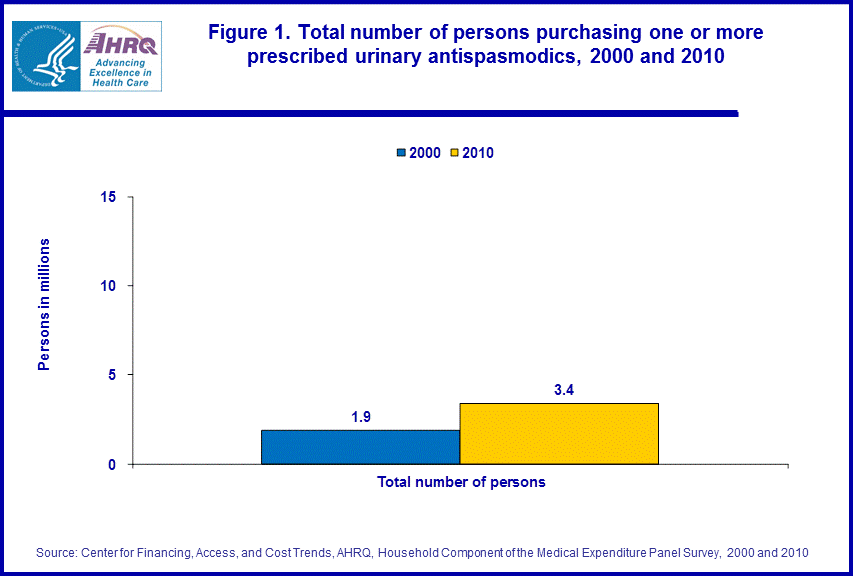
- From 2000 to 2010, the number of people in the U.S. civilian noninstitutionalized population obtaining at least one outpatient prescription urinary antispasmodic increased from 1.9 million people to 3.4 million people.
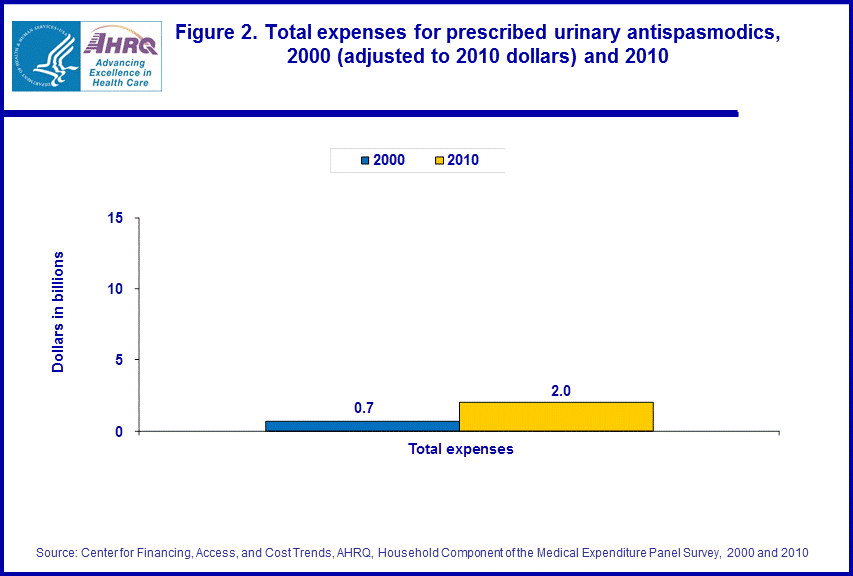
- From 2000 to 2010, inflation adjusted total expenses increased from $0.7 billion to $2.0 billion.
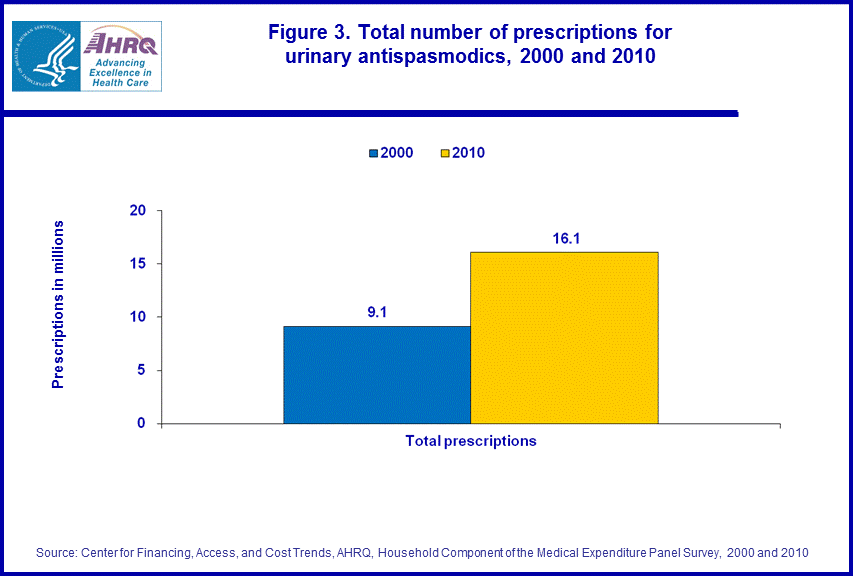
- From 2000 to 2010, utilization of outpatient prescription urinary antispasmodics increased from 9.1 million prescriptions to 16.1 million prescriptions.
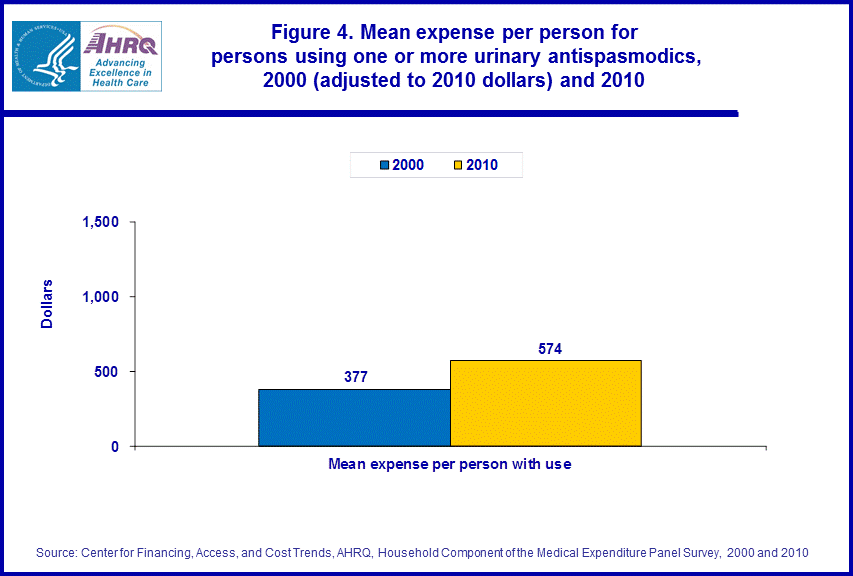
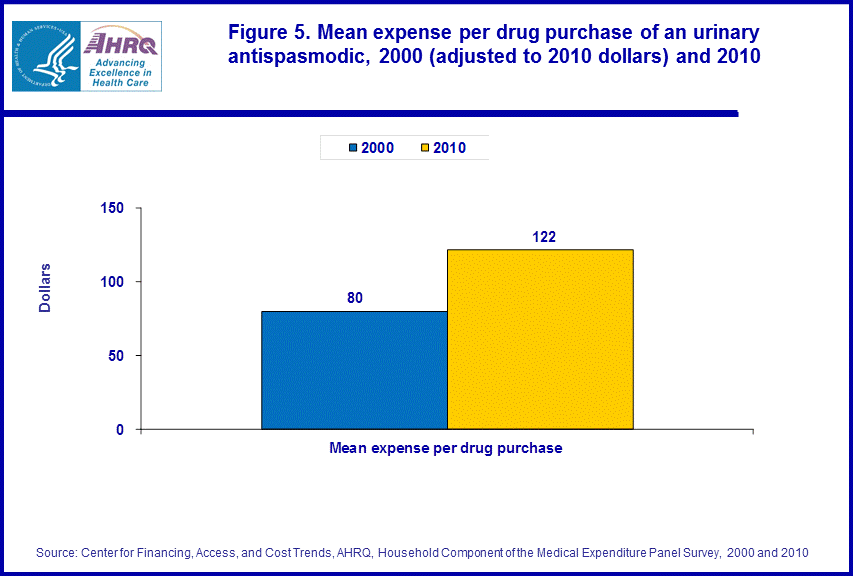
- From 2000 to 2010, the inflation adjusted average cost per drug purchase of a prescription urinary antispasmodic rose from $80 to $122.
Introduction
Rising health care costs in general and prescribed medicine costs in particular continue to be a concern for U.S. policymakers and consumers of care. Analyzing total prescription drug costs by therapeutic classes and subclasses provides decision makers and the public with an understanding of the costs and extent to which specific therapeutic classes and subclasses of drugs are contributing to the upturn in total costs.This Statistical Brief provides trends for one therapeutic subclass of prescribed drugs—urinary antispasmodics. This Brief presents trends in utilization and expenditures for outpatient prescription urinary antispasmodics for the years 2000 and 2010. The estimates are for the U.S. civilian noninstitutionalized population and are derived from the 2000 and 2010 Household Component of the Medical Expenditure Panel Survey (MEPS-HC). The Brief compares outpatient prescription urinary antispasmodics for 2000 and 2010, using the number of persons obtaining at least one prescription, total expenditures, and total number of prescriptions, as well as average annual cost per person and average drug cost.
Only prescriptions purchased or obtained in an outpatient setting are included in these estimates. Prescription medicines administered in an inpatient setting or in a clinic or physician's office are excluded. Expenditure estimates are presented in real dollars; estimates for 2000 were inflated to 2010 dollars based on the GDP Price Index (http://www.meps.ahrq.gov/mepsweb/about_meps/Price_Index.shtml). All differences discussed in the text are statistically significant at the 0.05 level.
Findings
Comparing 2000 with 2010, MEPS estimates showed an increase in the total number of people (as well as the proportion of the population) in the U.S. civilian noninstitutionalized population obtaining at least one urinary antispasmodic, rising from 1.9 million (0.7 percent of the 278.4 million people in the 2000 total population) to 3.4 million people (1.1 percent of the 308.6 million people in the 2010 total population) (figure 1).There was an increase of about 185 percent in total inflation adjusted expenditures for urinary antispasmodics when comparing the years 2000 ($0.7 billion) and 2010 ($2.0 billion) (figure 2).
From 2000 to 2010, MEPS estimates showed a growth in the total number of prescriptions for urinary antispasmodics from 9.1 million to 16.1 million prescriptions, an increase of 77 percent (figure 3).
There was an increase of 52.5 percent in the inflation adjusted average drug expense per purchase for a urinary antispasmodic when comparing 2000 with 2010, rising from $80 to $122 (figure 5).
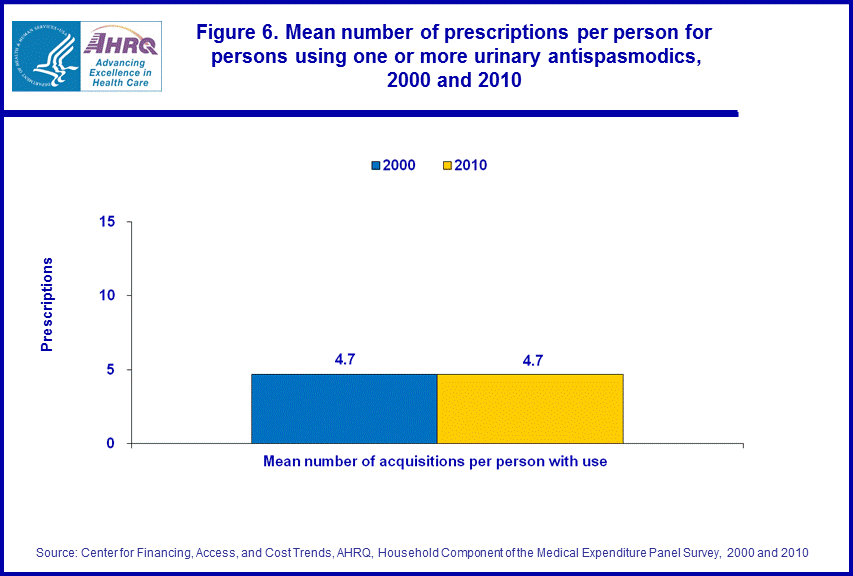
From 2000 to 2010, MEPS estimates showed no significant difference in the average number of prescriptions for urinary antispasmodics for those with one or more purchases (4.7 prescriptions in both 2000 and 2010) (figure 6).
Data Source
The estimates shown in this Statistical Brief are based on data from MEPS: HC-068: Multum Lexicon Addendum Files to MEPS Prescribed Medicines Files 1996–2001, HC-051A: 2000 Prescribed Medicines File, and HC-135A: 2010 Prescribed Medicines File.Definitions
Purchases and expendituresUtilization was defined as purchasing or obtaining urinary antispasmodics prescribed in the year of interest. Refills as well as original prescriptions are included in expenditure and utilization estimates. Expenditures include the total direct payments from all sources to pharmacies for prescriptions reported by respondents in the MEPS-HC. Expenditures are in real dollars; estimates for 2000 were adjusted to 2010 dollars based on the GDP Price Index (http://www.meps.ahrq.gov/mepsweb/about_meps/Price_Index.shtml).
Therapeutic classifications
Therapeutic class and subclass were assigned to MEPS prescribed medicines using Multum Lexicon variables from Cerner Multum, Inc. MEPS prescribed medicines files were linked to the Multum Lexicon database to obtain therapeutic class and subclass variables. The first choice in the linking algorithm was chosen when assigning therapeutic classes and subclasses. The following was used to define urinary antispasmodics: therapeutic class: central nervous system agents; subclass: urinary antispasmodics. In 2000 and 2010 common sub therapeutic subclasses for urinary antispasmodics included: barbiturate urinary antispasmodics, benzodiazepine urinary antispasmodics, and miscellaneous urinary antispasmodics. In 2000 only, the subclass urinary antispasmodics also included the following sub therapeutic subclass: hydantoin urinary antispasmodics. In 2010 only, the subclass urinary antispasmodics also included the following sub therapeutic subclasses: succinimide urinary antispasmodics, dibenzazepine urinary antispasmodics, fatty acid derivative urinary antispasmodics, gamma-aminobutyric acid analogs, triazine urinary antispasmodics, pyrrolidine urinary antispasmodics, and carbonic anhydrase inhibitor urinary antispasmodics. For additional information on these and other Multum Lexicon variables, please refer to the Multum Web site.
When looking at trends over time for therapeutic subclass and sub therapeutic subclasses, it is important to keep in mind many factors can play a role. These factors include: 1) drugs are reclassified due to changes in the Multum therapeutic classification scheme, 2) new drugs become available over time, and 3) generic versions of previously brand name only drugs become available.
About MEPS-HC
MEPS-HC is a nationally representative longitudinal survey that collects detailed information on health care utilization and expenditures, health insurance, and health status, as well as a wide variety of social, demographic, and economic characteristics for the U.S. civilian noninstitutionalized population. It is cosponsored by the Agency for Healthcare Research and Quality and the National Center for Health Statistics.MEPS expenditure data are derived from both the Medical Provider Component (MPC) and Household Component (HC). MPC data are generally used for hospital-based events (e.g., inpatient stays, emergency room visits, and outpatient department visits), prescribed medicine purchases, and home health agency care. Office-based physician care estimates use a mix of HC and MPC data while estimates for non-physician office visits, dental and vision services, other medical equipment and services, and independent provider home health care services are based on HC provided data. Details on the estimation process can be found in Machlin, S.R. and Dougherty, D.D. Overview of Methodology for Imputing Missing Expenditure Data in the Medical Expenditure Panel Survey. Methodology Report No. 19. March 2007. Agency for Healthcare Research and Quality, Rockville, MD. http://www.meps.ahrq.gov/mepsweb/data_files/publications/mr19/mr19.shtml
For more information about MEPS, call the MEPS information coordinator at AHRQ (301) 427-1656 or visit the MEPS Web site at http://www.meps.ahrq.gov/.
References
For a detailed description of the MEPS-HC survey design, sample design, and methods used to minimize sources of nonsampling error, see the following publications:Cohen, J. Design and Methods of the Medical Expenditure Panel Survey Household Component. MEPS Methodology Report No. 1. AHCPR Pub. No. 97-0026. Rockville, MD. Agency for Health Care Policy and Research, 2001. http://www.meps.ahrq.gov/mepsweb/data_files/publications/mr1/mr1.shtml
Cohen, S. Sample Design of the 1996 Medical Expenditure Panel Survey Household Component. MEPS Methodology Report No. 2. AHCPR Pub. No. 97-0027. Rockville, MD. Agency for Health Care Policy and Research, 2001. http://www.meps.ahrq.gov/mepsweb/data_files/publications/mr2/mr2.shtml
Cohen, S. Design Strategies and Innovations in the Medical Expenditure Panel Survey. Medical Care, July 2003: 41(7) Supplement: III-5–III-12.
Ezzati-Rice, T.M., Rohde, F., Greenblatt, J. Sample Design of the Medical Expenditure Panel Survey Household Component, 1998–2008. Methodology Report No. 22. March 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://www.meps.ahrq.gov/mepsweb/data_files/publications/mr22/mr22.shtml
Suggested Citation
Stagnitti, M.N. Trends in Urinary Antispasmodics Utilization and Expenditures for the U.S. Civilian Noninstitutionalized Population, 2000 and 2010. Statistical Brief #400. January 2013. Agency for Healthcare Research and Quality, Rockville, MD. http://www.meps.ahrq.gov/mepsweb/data_files/publications/st400/stat400.shtmlAHRQ welcomes questions and comments from readers of this publication who are interested in obtaining more information about access, cost, use, financing, and quality of health care in the United States. We also invite you to tell us how you are using this Statistical Brief and other MEPS data and tools and to share suggestions on how MEPS products might be enhanced to further meet your needs. Please e-mail us at MEPSProjectDirector@ahrq.hhs.gov or send a letter to the address below:
Steven B. Cohen, PhD, Director
Center for Financing, Access, and Cost Trends
Agency for Healthcare Research and Quality
540 Gaither Road
Rockville, MD 20850
 |
||||||||||
|
||||||||||
|
|
||||||||||
 |
||||||||||
|
||||||||||
|
|
||||||||||
 |
||||||||||
|
||||||||||
|
|
||||||||||
 |
||||||||||
|
||||||||||
|
|
||||||||||
 |
||||||||||
|
||||||||||
|
|
||||||||||
 |
||||||||||
|
||||||||||
|
|
||||||||||


