MEPS HC-118A: 2008 Prescribed Medicines
October 2010
Agency for Healthcare Research and Quality
Center for Financing, Access, and Cost Trends
540 Gaither Road
Rockville, MD 20850
(301) 427-1406
Table of Contents
A. Data Use Agreement
B. Background
1.0 Household Component
2.0 Medical Provider Component
3.0 Survey Management and Data Collection
C. Technical Information
1.0 General Information
2.0 Data File Information
2.1 Using MEPS Data for Trend and Longitudinal Analysis
2.2 Codebook Structure
2.3 Reserved Codes
2.4 Codebook Format
2.5 Variable Naming Conventions
2.5.1 General
2.5.2 Expenditure and Source of Payment Variables
2.6 Data Collection
2.6.1 Methodology for Collecting Household Reported
Variables
2.6.2 Methodology for Collecting Pharmacy Reported
Variables
2.7 File Contents
2.7.1 Survey Administration Variables
2.7.1.1 Person Identifier Variables (DUID, PID, DUPERSID)
2.7.1.2 Record Identifier Variables (RXRECIDX, LINKIDX)
2.7.1.3 Panel Variable (PANEL)
2.7.1.4 Round Variable (PURCHRD)
2.7.1.5 Duplicate Purchase (DUP2007)
2.7.2 Characteristics of Prescribed Medicine Events
2.7.2.1 Date When Prescribed Medicine Was First Taken (RXBEGMM-RXBEGYRX)
2.7.2.2 Prescribed Medicine Attributes (RXNAME-RXSTRUNT)
2.7.2.3 Type of Pharmacy (PHARTP1-PHARTP17)
2.7.2.4 Analytic Flag Variables (RXFLG-INPCFLG)
2.7.2.5 The Sample Variable (SAMPLE)
2.7.2.6 Condition Codes (RXICD1X-RXICD3X) and Clinical
Classification Codes (RXCCC1X-RXCCC3X)
2.7.3 Multum Lexicon Variables from Cerner Multum, Inc.
2.7.4 Expenditure Variables (RXSF08X-RXXP08X)
2.7.4.1 Definition of Expenditures
2.7.4.2 Sources of Payment
2.7.5 Sample Weight (PERWT08F)
2.7.5.1 Overview
2.7.5.2 Details on Person Weights Construction
2.7.5.3 MEPS Panel 12 Weight
2.7.5.4 MEPS Panel 13 Weight
2.7.5.5 The Final Weight for 2008
2.7.5.6 Additional Adjustment to 2008 Person Weights for Persons Age 65 and Over
2.7.5.7 Coverage
3.0 General Data Editing and Imputation Methodology
3.1 Rounding
3.2 Edited/Imputed Expenditure Variables (RXSF08X-RXXP08X)
4.0 Strategies for Estimation
4.1 Developing Event-Level Estimates
4.2 Person-Based Estimates for Prescribed Medicine Purchases
4.3 Variables with Missing Values
4.4 Variance Estimation (VARSTR, VARPSU)
5.0 Merging/Linking MEPS Data Files
5.1 Linking to the Person-Level File
5.2 Linking to the Medical Conditions File
5.3 Pooling Annual Files
5.4 Longitudinal Analysis
References
D. Variable-Source Crosswalk
Attachment 1 Definitions of Abbreviations for RXFORM
Attachment 2 Definitions of Codes and Abbreviations for
RXFRMUNT
Attachment 3 Definitions of Abbreviations, Codes and Symbols
for RXSTRUNT
Attachment 4 Theraputic Class Code Definitions
A. Data Use Agreement
Individual identifiers have been removed from the micro-data
contained in these files. Nevertheless, under sections 308 (d) and 903 (c)
of the Public Health Service Act (42 U.S.C. 242m and 42 U.S.C. 299 a-1), data
collected by the Agency for Healthcare Research and Quality (AHRQ) and/or the
National Center for Health Statistics (NCHS) may not be used for any purpose
other than for the purpose for which they were supplied; any effort to determine
the identity of any reported cases is prohibited by law.
Therefore in accordance with the above referenced Federal Statute, it is understood
that:
- No one is to use the data in this data set in any way except for statistical
reporting and analysis; and
- If the identity of any person or establishment should be discovered
inadvertently, then (a) no use will be made of this knowledge, (b) the
Director Office of
Management AHRQ will be advised of this incident, (c) the information
that would identify any individual or establishment will be safeguarded
or destroyed,
as requested by AHRQ, and (d) no one else will be informed of the discovered
identity; and
- No one will attempt to link this data set with individually identifiable
records from any data sets other than the Medical Expenditure Panel
Survey or the National Health Interview Survey.
By using these data you
signify your agreement to comply with the above stated statutorily based
requirements with the knowledge that
deliberately making
a false statement in any matter within the jurisdiction of
any department or agency of the Federal Government violates Title 18 part
1 Chapter
47 Section
1001 and is punishable by a fine of up to $10,000 or up to
5 years
in prison. The Agency for Healthcare Research and Quality requests that users cite AHRQ
and the Medical Expenditure Panel Survey as the data source in any publications
or research based upon these data.
Return To Table Of Contents
B. Background
1.0 Household Component (HC)
The Medical Expenditure Panel Survey (MEPS) provides
nationally representative estimates of health care use, expenditures, sources
of payment, and health insurance coverage for the U.S. civilian non-institutionalized
population. The MEPS Household Component (HC) also provides estimates of respondents'
health status, demographic and socio-economic characteristics, employment,
access to care, and satisfaction with health care. Estimates can be produced
for individuals, families, and selected population subgroups. The panel design
of the survey, which includes 5 Rounds of interviews covering 2 full calendar
years, provides data for examining person level changes in selected variables
such as expenditures, health insurance coverage, and health status. Using computer
assisted personal interviewing (CAPI) technology, information about each household
member is collected, and the survey builds on this information from interview
to interview. All data for a sampled household are reported by a single household
respondent.
The MEPS-HC was initiated in 1996. Each year a new panel of households is
selected. Because the data collected are comparable to those from earlier medical
expenditure surveys conducted in 1977 and 1987, it is possible to analyze long-term
trends. Each annual MEPS-HC sample size is about 15,000 households. Data can
be analyzed at either the person or event level. Data must be weighted to produce
national estimates.
The set of households selected for each panel of the MEPS HC is a subsample
of households participating in the previous year's National Health Interview
Survey (NHIS) conducted by the National Center for Health Statistics. The NHIS
sampling frame provides a nationally representative sample of the U.S. civilian
non-institutionalized population and reflects an oversample of blacks and Hispanics.
In 2006, the NHIS implemented a new sample design, which included Asian persons
in addition to households with black and Hispanic persons in the oversampling
of minority populations. MEPS oversamples additional policy relevant sub-groups
such as Asians and low income households. The linkage of the MEPS to the previous
year's NHIS provides additional data for longitudinal analytic purposes.
Return To Table Of Contents
2.0 Medical Provider Component (MPC)
Upon completion of the household CAPI interview and obtaining
permission from the household survey respondents, a sample of medical providers
are contacted by telephone to obtain information that household respondents
can not accurately provide. This part of the MEPS is called the Medical Provider
Component (MPC) and information is collected on dates of visit, diagnosis and
procedure codes, charges and payments. The Pharmacy Component (PC), a subcomponent
of the MPC, does not collect charges or diagnosis and procedure codes but does
collect drug detail information, including National Drug Code (NDC) and medicine
name, as well as date filled and sources and amounts of payment. The MPC is
not designed to yield national estimates. It is primarily used as an imputation
source to supplement/replace household reported expenditure information.
Return To Table Of Contents
3.0 Survey Management and Data Collection
MEPS HC and MPC data are collected under the authority
of the Public Health Service Act. Data are collected under contract with Westat,
Inc. Data sets and summary statistics are edited and published in accordance
with the confidentiality provisions of the Public Health Service Act and the
Privacy Act. The National Center for Health statistics (NCHS) provides consultation
and technical assistance.
As soon as data collection and editing are completed, the MEPS survey data
are released to the public in staged releases of summary reports, micro data
files, and tables via the MEPS web site: www.meps.ahrq.gov. Selected
data can be analyzed through MEPSnet, an on-line interactive tool designed
to give
data users the capability to statistically analyze MEPS data in a menu-driven
environment.
Additional information on MEPS is available from the MEPS project manager or
the MEPS public use data manager at the Center for Financing Access and Cost
Trends, Agency for Healthcare Research and Quality, 540 Gaither Road, Rockville,
MD 20850 (301-427-1406).
Return To Table Of Contents
C. Technical
Information
1.0 General Information
This documentation describes one in a series of public
use event files from the 2008 Medical Expenditure Panel Survey (MEPS) Household
Component (HC) and Medical Provider Component (MPC). Released as an ASCII data
file(with related SAS and SPSS programming statements) and SAS transport file,
the 2008 Prescribed Medicines public use file provides detailed information
on household reported prescribed medicines for a nationally representative
sample of the civilian noninstitutionalized population of the United States.
Data from the Prescribed Medicines event file can be used to make estimates
of prescribed medicine utilization and expenditures for calendar year 2008.
The file contains 77 variables and has a logical record length of 531 with
an additional 2-byte carriage return/line feed at the end of each record. As
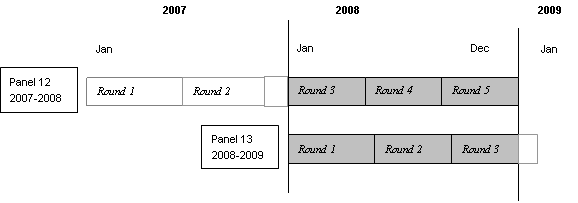
illustrated below, this file consists of MEPS survey data obtained in the 2008
portion of Round 3 and Rounds 4 and 5 for Panel 12, as well as Rounds 1, 2
and the 2008 portion of Round 3 for Panel 13 (i.e., the rounds for the MEPS
panels covering calendar year 2008).
Incentive Experiment in Panel 13
With the encouragement of the Office of Management and Budget (OMB), an experiment
was undertaken for MEPS Panel 13 (first fielded in 2008) to evaluate whether
and how differential payments to household respondents might affect survey
participation, the level of effort required to obtain participation, and the
quality of the data collected. Each sampled household in Panel 13 was randomly
assigned to one of three different levels of payment—$30, $50, or $70—with
the experiment continuing through the panel’s five rounds of data collection.
Households receiving the $30 payment represent the control group, since that
amount had been offered to all households in the 2007 panel. To learn more
about this experiment, refer to the Household Annual Contractor Methodology Report (located in the Household—Survey Basics section). Agency for Healthcare Research and Quality, Rockville, MD.
Each record on this event file represents a unique prescribed medicine event;
that is, a prescribed medicine reported as being purchased by the household
respondent. In addition to expenditures related to the prescribed medicine,
each record contains household reported characteristics and medical conditions
associated with the prescribed medicine.
Data from this event file can be merged with other 2008 MEPS-HC data files,
for purposes of appending person characteristics such as demographic or health
insurance coverage to each prescribed medicine record.
Counts of prescribed medicine utilization are based entirely on household
reports. Information from the Pharmacy Component (PC) (within the MEPS-MPC,
see section B.2.0 for more details on the MPC) was used to provide expenditure
and payment data, as well as details of the medication (e.g., strength, quantity,
etc.).
The file can be used to construct summary variables of expenditures, sources
of payment, and other aspects of utilization of prescribed medicines. Aggregate
annual person-level information on the use of prescribed medicines and other
health services use is provided on the 2008 Full Year Consolidated Data File,
where each record represents a MEPS sampled person.
The following documentation offers a brief overview of the types and levels
of data provided and the content and structure of the files and the codebook.
It contains the following sections:
Data File Information
Sample Weight
General Data Editing and Imputation Methods
Strategies for Estimation
Merging/Linking MEPS Data Files
References
Variable to Source Crosswalk
For more information on MEPS-HC survey design see S. Cohen, 1997; J. Cohen,
1997; and S. Cohen, 1996. For information on the MEPS-MPC design, see S. Cohen,
1998. A copy of the survey instrument used to collect the information on this
file is available on the MEPS Web site at the following address: www.meps.ahrq.gov.
Return To Table Of Contents
2.0 Data File Information
The 2008 Prescribed Medicines public use data set contains
293,379 prescribed medicine records. Each record represents one household reported
prescribed medicine that was purchased during calendar year 2008. Of the 293,379
prescribed medicine records, 287,606 records are associated with persons having
a positive person-level weight (PERWT08F). The persons represented on this
file had to meet either criterion a or b below:
- Be classified as a key in-scope person who responded for his
or her entire period of 2008 eligibility (i.e., persons with a positive 2008
full-year
person-level
sampling weight (PERWT08F > 0), or
- Be an eligible member of a family all of whose key in-scope members
have a positive person-level weight (PERWT08F > 0). (Such a family consists of
all persons with the same value for FAMIDYR.) That is, the person must have
a positive full-year family-level weight (FAMWT08F >0). Note that FAMIDYR
and FAMWT08F are variables on the 2008 Population Characteristics file.
Persons with no prescribed medicine use for 2008 are not included on this
file (but are represented on MEPS person-level files). A codebook for the data
file is provided (in file H118ACB.PDF).
This file includes prescribed medicine records for all household survey respondents
who resided in eligible responding households and reported at least one prescribed
medicine. Only prescribed medicines that were purchased in calendar year 2008
are represented on this file. This file includes prescribed medicines identified
in the Prescribed Medicines section of the HC survey instrument, as well as
those prescribed medicines identified in association with other medical events.
Each record on this file represents a single acquisition of a prescribed medicine
reported by household respondents. Some household respondents may have multiple
acquisitions of prescribed medicines and thus will be represented in multiple
records on this file. Other household respondents may have reported no acquisitions
of prescribed medicines and thus will have no records on this file.
When diabetic supplies, such as syringes and insulin, were mentioned in the
Other Medical Equipment section of the MEPS-HC, the interviewer was directed
to collect information on these items in the Prescription Medicines section
of the MEPS questionnaire. The respondent was also asked the questions in the
Charge and Payment section of the HC. To the extent that these items are purchased
without a prescription, they represent a non-prescription addition to the MEPS
prescription drug expenditure and utilization data. Although these items may
be purchased without a prescription, a prescription purchase may be required
to obtain third party payments. Analysts are free to code and define diabetic
supply/equipment and insulin events utilizing their own coding mechanism. If
desired, this would enable analysts to subset the Prescribed Medicines file
to exclude these types of events.
It should also be noted that refills are included on this file. The HC obtains
information on the name of the prescribed medicine and the number of times
the medicine was obtained. The data collection design for the HC does not allow
separate records to be created for multiple acquisitions of the same prescribed
medicine. However, in the PC, each original purchase, as well as any refill,
is considered a unique prescribed medicine event. Therefore, for the purposes
of editing, imputation and analysis, all records in the HC were “unfolded” to
create separate records for each original purchase and each refill. Please
note: MEPS did not collect information in the HC to distinguish multiple acquisitions
of the same drug between the original purchase and refills. The survey only
collected data on the number of times a prescribed medicine was acquired during
a round. In some cases, all purchases may have been refills of an original
purchase in a prior round or prior to the survey year. The file also includes
a variable, (SAMPLE), which indicates whether or not the household reported
receiving a free sample of that drug in that round. (To obtain more details
on free samples, please see section 2.7.2.5.)
Each record on this file includes the following: an identifier for each unique
prescribed medicine; detailed characteristics associated with the event (e.g.,
national drug code (NDC), medicine name, selected Multum Lexicon variables
[see section 2.7.3 for more information on the Multum Lexicon variables included
on this file], etc.); conditions, if any, associated with the medicine; the
date on which the person first used the medicine; total expenditure and sources
of payments; types of pharmacies that filled the household’s prescriptions;
whether the prescription is one in which the household received a free sample
of it during the round; and a full-year person-level weight.
Data from this file can be merged with previously released MEPS-HC person-level
data using the unique person identifier, DUPERSID, to append person characteristics
such as demographic or health insurance coverage to each record. Data from
this file can also be merged with the 2008 Full Year Consolidated Data File
to estimate expenditures for persons with prescribed medicines. The Prescribed
Medicines event file can also be linked to the MEPS 2008 Medical Conditions
File and additional MEPS 2008 event files. Please see the 2008 Appendix File
for details on how to link MEPS data files.
Return To Table Of Contents
2.1 Using MEPS Data for
Trend and Longitudinal Analysis
MEPS began in 1996 and several annual data files have
been released. As more years of data are produced, MEPS will become increasingly
valuable for examining health care trends. However, it is important to consider
a variety of factors when examining trends over time using MEPS. Statistical
significance tests should be conducted to assess the likelihood that observed
trends are attributable to sampling variation. MEPS expenditures estimates
are especially sensitive to sampling variation due to the underlying skewed
distribution of expenditures. For example, 1 percent of the population accounts
for about one-quarter of all expenditures. The extent to which observations
with extremely high expenditures are captured in the MEPS sample varies from
year to year (especially for smaller population subgroups), which can produce
substantial shifts in estimates of means or totals that are simply an artifact
of the sample(s). The length of time being analyzed should also be considered.
In particular, large shifts in survey estimates over short periods of time
(e.g., from one year to the next) that are statistically significant should
be interpreted with caution, unless they are attributable to known factors
such as changes in public policy or MEPS survey methodology. In particular,
beginning with the 2007 data, the rules used to identify outlier prices for
prescription medications became much less stringent than in prior years. Starting
with the 2007 Prescribed Medicine file, there is less editing of prices and
quantities reported by pharmacies, more variation in prices for generics, lower
mean prices for generics, higher mean prices for brand name drugs, greater
differences in prices between generic and brand name drugs, and a somewhat
lower proportion of spending on drugs is by families, as opposed to third-party
payers. Starting with the 2008 Prescribed Medicine file, improvements in the
data editing changed the distribution of payments by source: (1) more spending
on Medicare beneficiaries is by private insurance, rather than Medicare, and
(2) less out-of-pocket payments and more Medicaid payments among Medicaid enrollees.
Therefore users should be cautious in the types of comparisons they make about
prescription drug spending before and after 2007 and 2008. For other time periods
or other characteristics of prescription drugs, looking at changes over longer
periods of time can provide a more complete picture of underlying trends. Analysts
may wish to consider using techniques to smooth or stabilize trends analyses
of MEPS data such as pooling time periods for comparison (e.g., 1996-97 versus
1998-99), working with moving averages, or using modeling techniques with several
consecutive years of MEPS data to test the fit of specified patterns over time.
Finally, researchers should be aware of the impact of multiple comparisons
on Type I error because performing numerous statistical significance tests
of trends increases the likelihood of inappropriately concluding a change is
statistically significant.
The records on this file can be linked to all other 2008 MEPS-HC public use
data sets by the sample person identifier (DUPERSID). Panel 12 cases (PANEL=12)
can be linked back to the 2007 MEPS-HC public use files by DUPERSID and the
panel indicator (PANEL).
Return To Table Of Contents 2.2 Codebook Structure
For each variable on the file, both weighted and unweighted
frequencies are provided. The codebook and data file sequence list variables
in the following order:
Unique person identifiers
Unique prescribed medicine identifiers
Other survey administration variables
Prescribed medicine characteristics variables
ICD-9 codes for medical conditions
Clinical Classification Software codes for medical conditions
Multum Lexicon variables
Expenditure variables
Weight and variance estimation variables
Return To Table Of Contents
2.3 Reserved Codes
The following reserved code values are used:
|
Value
|
Definition
|
| -1 INAPPLICABLE |
Question was not asked due to skip pattern. |
| -7 REFUSED |
Question was asked and respondent refused to answer question. |
| -8 DK |
Question was asked and respondent did not know answer. |
| -9 NOT ASCERTAINED |
Interviewer did not record the data. |
| -14 NOT YET TAKEN/USED |
Respondent answered that the medicine has not yet been used. |
Generally, values of -1, -7, -8 and -9 have not been edited on this file.
However, this is not true if the pharmacist determined a prescription drug
name to be a confidentiality risk. In these instances, generally, the corresponding
NDC was replaced with -9, and the Multum Lexicon therapeutic class was the
replacement for the drug name determined a confidentiality risk. The values
of -1 and -9 can be edited by analysts by following the skip patterns in the
questionnaire. The value -14 was a valid value only for the variable representing
the year the respondent reported having first used the medicine (RXBEGYRX).
RXBEGYRX= -14 means that when the interviewer asked the respondent the year
he/she first started using the medicine, he/she responded that he/she had not
yet started using the medicine. A copy of the Household Component questionnaire can be found on the World
Wide Web at
www.meps.ahrq.gov/survey_comp/survey.jsp by selecting Prescribed
Medicines (PM) from the questionnaire section.
Return To Table Of Contents
2.4 Codebook Format
The codebook describes an ASCII data set (although the
data are also being provided in a SAS transport file). The following codebook
items are provided for each variable:
|
Identifier
|
Definition
|
| Name |
Variable name (maximum of 8 characters) |
| Description |
Variable descriptor (maximum of 40 characters) |
| Format |
Number of bytes |
| Type |
Type of data: numeric (indicated by NUM) or
character (indicated by CHAR) |
| Start |
Beginning column position of variable in record |
| End |
Ending column position of variable in record |
Return To Table Of Contents
2.5 Variable
Naming Conventions
In general, variable names reflect the content of the
variable, with an eight-character limitation. Generally, imputed/edited variables
end with an “X.”
Return To Table Of Contents
2.5.1 General
Variables contained on this file were derived from the
HC questionnaire itself, the MPC data collection instrument, the CAPI, or from
the Multum Lexicon database from Cerner Multum, Inc. The source of each variable
is identified in section D, entitled “Variable-Source Crosswalk.” Sources
for each variable are indicated in one of four ways: (1) variables which are
derived from CAPI or assigned in sampling are so indicated; (2) variables which
come from one or more specific questions have those numbers and the questionnaire
section indicated in the “Source” column; (3) variables constructed
from multiple questions using complex algorithms are labeled “Constructed” in
the “Source” column; (4) variables which have been imputed are
so indicated; and (5) variables derived from the Multum Lexicon database are
so indicated.
Return To Table Of Contents
2.5.2 Expenditure and
Source of Payment Variables
Only imputed/edited versions of the expenditure variables
are provided on the file. Expenditure variables on this event file follow a
standard naming convention and are 7 characters in length. The 12 source of
payment variables and one sum of payments variable are named consistently in
the following way:
The first two characters indicate the type of event:
IP - inpatient stay
ER - emergency room visit
HH - home health visit
OM - other medical equipment
OB - office-based visit
OP
- outpatient visit
DV - dental visit
RX - prescribed medicine
In the case of the source of payment variables, the third and fourth characters
indicate:
SF - self or family
MR - Medicare
MD - Medicaid
PV - private insurance
VA - Veterans
TR - TRICARE
OF - other Federal Government
SL - State/local government
WC - Worker’s
Compensation
OT - other insurance
OR - other private
OU - other public
XP - sum
of payments
The fifth and sixth characters indicate the year (08). The seventh character, “X”,
indicates the variable is edited/imputed.
For example, RXSF08X is the edited/imputed amount paid by self or family for
the 2008 prescribed medicine expenditure.
Return To Table Of Contents 2.6 Data Collection
Data regarding prescription drugs were obtained through the HC questionnaire
and a pharmacy follow-back component (within the Medical Provider Component).
2.6.1 Methodology for Collecting Household Reported Variables
During each round of the MEPS-HC, all respondents were asked to supply the
name of any prescribed medicine they or their family members purchased or otherwise
obtained during that round. For each medicine in each round, the following
information was collected: whether any free samples of the medicine were received;
the name(s) of any health problems the medicine was prescribed for; the number
of times the prescription medicine was obtained or purchased; the year and
month on which the person first used the medicine; and a list of the names,
addresses, and types of pharmacies that filled the household’s prescriptions.
In the HC, respondents were asked if they send in claim forms for their prescriptions
or if their pharmacy providers do this automatically for them at the point
of purchase. For those who said their pharmacy providers automatically send
in claims for them at the point of purchase, charge and payment information
was collected in the pharmacy follow-back component (unless the purchase was
an insulin or diabetic supply/equipment event that was mentioned in the household
component; see section 3.0 for details). However, charge and payment information
was collected for those who said they send in their own prescription claim
forms, because it was thought that payments by private third-party payers for
those who filed their own claim forms for prescription purchases would not
be available from pharmacies. Uninsured persons were treated in the same manner
as those whose pharmacies filed their prescription claims at the point of purchase.
Persons who said they did not know if they sent in their own prescription claim
forms were treated as those who said they did send in their own prescription
claim forms.
In consultation with an industry expert, outlier values for the number of
times a household reported purchasing or otherwise obtaining a prescription
drug in a particular round were determined by comparing the number of days
a respondent was in the round and the number times the person reported obtaining
the drug in the round. For these events, a new value for the number of times
a drug was purchased or otherwise obtained by a person in a round was imputed.
In addition, the prescribed medicine events in which a household respondent
did not know/remember the number of times a certain prescribed medicine was
purchased or otherwise obtained were imputed a value for that variable.
For those rounds that spanned two years, drugs mentioned in that round were
allocated between the years based on the number of times the respondent said
the drug was purchased in the respective year, the year the person started
taking the drug, the length of the person’s round, the dates of the person’s
round, and the number of drugs for that person in the round. In addition, a “folded” version
of the PC on a drug level, as opposed to an acquisition level, was used for
these types of events to assist in determining how many acquisitions of the
drug should be allocated between the years.
Return To Table Of Contents 2.6.2 Methodology for Collecting Pharmacy Reported Variables
If the respondent with the prescription gave written permission to release
his or her pharmacy records, pharmacy providers identified by the household
were contacted by telephone for the pharmacy follow-back component. Following
an initial telephone contact, the signed permission forms and materials explaining
the study were faxed (or mailed) to cooperating pharmacy providers. The materials
informed the providers of all persons participating in the survey who had prescriptions
filled at their place of business and requested a computerized printout of
all prescriptions filled for each person. For each medication listed, the following
information was requested: date filled; national drug code (NDC); medication
name; strength of medicine (amount and unit); quantity (package size/amount
dispensed); and payments by source.
Return To Table Of Contents 2.7 File Contents
2.7.1 Survey Administration Variables
2.7.1.1 Person Identifier Variables (DUID, PID, DUPERSID)
The dwelling unit ID (DUID) is a five-digit random number assigned after the
case was sampled for MEPS. The three-digit person number (PID) uniquely identifies
each person within the dwelling unit. The eight-character variable DUPERSID
uniquely identifies each person represented on the file and is the combination
of the variables DUID and PID. For detailed information on dwelling units and
families, please refer to the documentation for the 2008 Full Year Population
Characteristics File.
Return To Table Of Contents 2.7.1.2 Record Identifier Variables (RXRECIDX, LINKIDX)
The variable RXRECIDX uniquely identifies each record on the file. This 15-character
variable is comprised of the following components: prescribed medicine drug-level
identifier generated through the HC (positions 1-12) + enumeration number (positions
13-15). The prescribed medicine drug-level ID generated through the HC (positions
1-12) can be used to link a prescribed medicine event to the conditions file
and to other event files, via link files, and is provided on this file as the
variable LINKIDX. (For more details on linking, please refer to section 5.2
and to the 2008 Appendix File.)
The following hypothetical example illustrates the structure of these ID variables.
This example illustrates a person in Round 1 of the household interview who
reported having purchased Amoxicillin three times. The following example shows
three acquisition-level records, all having the same RXNDC (00093310905), for
one person (DUPERSID=00002026) in one round. Generally, one NDC is associated
with a prescribed medicine event because matching was performed at a drug level,
as opposed to an acquisition level. The LINKIDX (000020260083) remains the
same for all three records, whereas the RXRECIDX (000020260083001, 000020260083002,
000020260083003) differs for all three records.
| DUPERSID |
RXRECIDX |
LINKIDX |
RXNDC |
| 00002026 |
000020260083001 |
000020260083 |
00093310905 |
| 00002026 |
000020260083002 |
000020260083 |
00093310905 |
| 00002026 |
000020260083003 |
000020260083 |
00093310905 |
Starting with the 2008 Prescribed Medicine file, there can be multiple RXNDCs
for a LINKIDX. All the acquisitions in the LINKID represent the same drug (active
ingredients), but the RXNDCs may represent different manufacturers. (For more
details on matching, please see section 3.0).
Return To Table Of Contents 2.7.1.3 Panel Variable (PANEL)
PANEL is a constructed variable used to specify the panel number for the person.
Panel will indicate either Panel 12 or Panel 13 for each person on the file.
Panel 12 is the panel that started in 2007, and Panel 13 is the panel that
started in 2008.
2.7.1.4 Round Variable (PURCHRD)
The variable PURCHRD indicates the round in which the prescribed medicine
was purchased and takes on the value of 1, 2, 3, 4, or 5. Rounds 3, 4, and
5 are associated with MEPS survey data collection from Panel 12. Similarly,
Rounds 1, 2, and 3 are associated with data collected from Panel 13.
2.7.1.5 Duplicate Purchase (DUP2007)
Starting with the 2008 Prescription Medicine file, the method used to allocate
some round 3 records between years was changed. This change does not affect
analyses using the full year file, because it was implemented for both panels.
However, for users conducting analyses of Panel 12 that use both the 2007 and
the 2008 drug data, the variable DUP2007 identifies records on the 2008 Prescription
Medicine file that duplicate acquisitions on the 2007 Prescription Medicine
file.
Return To Table Of Contents
2.7.2 Characteristics of Prescribed Medicine Events
2.7.2.1 Date When Prescribed Medicine Was First Taken (RXBEGMM-RXBEGYRX)
There are two variables which indicate when a prescribed medicine was first
taken (used), as reported by the household. They are the following: RXBEGMM
denotes the month in which a person first started taking a medication, and
RXBEGYRX reflects the year in which a person first started taking a medicine.
These “first taken” questions are only asked the first time a prescription
is mentioned by the household. These questions are not asked of refills of
the prescription for a person in subsequent rounds and result in a value of
-1 being assigned to those types of events for these variables. For purposes
of confidentiality, RXBEGYRX was bottom-coded at 1923 which makes RXBEGYRX
consistent with the top-coding of the age variables on the 2008 Full Year Population
Characteristics Public Use File (HC-115).
2.7.2.2 Prescribed Medicine Attributes (RXNAME-RXSTRUNT)
For each prescribed medicine included on this file, several data items collected
describe in detail the medication obtained or purchased. These data items are
the following:
- Medication name - pharmacy reported (RXNAME)
- National drug code (RXNDC)
- Quantity of the prescribed medicine dispensed (RXQUANTY); e.g., number
of tablets in the prescription
- Form of the prescribed medicine (RXFORM); e.g., powder
- Unit of measurement for form of Rx/prescribed medicine (RXFRMUNT);
e.g., oz
- Strength of the dose of the prescribed medicine (RXSTRENG); e.g.,
10
- Unit of measurement for the strength of the dose of the prescribed
medicine (RXSTRUNT); e.g., gm
Please refer to Attachments 1, 2, and 3 for definitions for RXFORM, RXFRMUNT,
and RXSTRUNT abbreviations, codes and symbols. Please refer to Attachment 4
for therapeutic class code definitions.
The national drug code (NDC) generally, and on this file, is an 11-digit code.
The first 5 digits indicate the manufacturer of the prescribed medicine. The
next 4 digits indicate the form and strength of the prescription, and the last
2 digits indicate the package size from which the prescription was dispensed.
NDC values were imputed from a proprietary database to certain PC prescriptions
because the NDC reported by the pharmacy provider was not valid. These records
are identified by RXFLG=3.
For the years 1996-2004, AHRQ’s licensing agreement with the proprietary
database precluded the release of the imputed NDC values to the public, so
for these prescriptions, the household reported name of the prescription (RXHHNAME)
and the original NDC (RXNDC) and prescription name (RXNAME) reported by the
pharmacy were provided on the file to allow users to do their own imputation.
In addition, for the years 1996-2004, the imputed NDC values for the RXFLG=3
cases could be accessed through the MEPS Data Center. For those events not
falling in the RXFLG=3 category, the reserve code (-13) was assigned to the
household reported medication name (RXHHNAME). The household reported name
of the prescription (RXHHNAME) is no longer provided on this file; however,
this variable may be accessed through the MEPS Data Center as can the original
pharmacy reported name and NDC. For information on accessing data through the
MEPS Data Center, see the Data Center section of the MEPS Web site at:
www.meps.ahrq.gov/data_stats/onsite_datacenter.jsp.
Imputed data on this event file, unlike other MEPS event files, may still
have missing data. This is because imputed data on this file are imputed
from the PC or from a proprietary database. These sources did not always
include complete information for each variable but did include an NDC, which
would typically enable an analyst to obtain any missing data items. For example,
although there are a substantial number of missing values for the strength
of the prescription that were not supplied by the pharmacist, these missing
values were not imputed because this information is embedded in the NDC.
Return To Table Of Contents 2.7.2.3 Type of Pharmacy (PHARTP1-PHARTP17)
Household respondents were asked to list the type of pharmacy from which their
medications were purchased. A household could list multiple pharmacies associated
with their prescriptions in a given round or over the course of all rounds
combined covering the survey year. All household reported pharmacies are provided
on this file, but there was no link in the survey or in the data file enabling
users to know the type of pharmacy from which a specific prescription was obtained
if multiple pharmacies are listed. The set of variables (PHARTP1-PHARTP17)
identify the types of pharmacy providers from which the person’s prescribed
medicines were purchased. The possible types of pharmacies include the following:
(1) mail-order, (2) another store, (3) HMO/clinic/hospital, (4) drug store,
and (5) on-line. A -1 value for PHARTPn indicates that the household did not
report an “nth” pharmacy.
2.7.2.4 Analytic Flag Variables (RXFLG-INPCFLG)
There are four flag variables included on this file (RXFLG, PCIMPFLG, CLMOMFLG,
and INPCFLG).
The variable RXFLG indicates how the NDC for a specific prescribed medicine
event was imputed. This variable indicates whether or not there was any imputation
performed on this record for the NDC variable, and if imputed, from what source
the NDC was imputed. If no imputation was performed, RXFLG=1. If the imputation
source was another PC record, RXFLG=2. Similarly, if the imputation source
was a secondary, proprietary database and not the PC database, RXFLG=3.
PCIMPFLG indicates the type of match between a household reported event and
a PC reported event. There are only two possible values for this variable (PCIMPFLG
=1 or =2). These values indicate the possible “match-types” and
are the following: =1 is an exact match for a specific event for a person between
the PC and the HC and =2 is not an exact match between the PC and HC for a
specific person (not an exact match means that a person’s household reported
event did not have a matched counterpart in their corresponding PC records).
PCIMPFLG assists analysts in determining which records have the strongest link
to data reported by a pharmacy. It should be noted that whenever there are
multiple purchases of a unique prescribed medication in a given round, MEPS
did not collect information that would enable designating any single purchase
as the “original” purchase at the time the prescription was first
filled, and then designating other purchases as “refills.” The
user needs to keep this in mind when the purchases of a medication are referred
to as “refills” in the documentation. Because matching was performed
at a drug level as opposed to an acquisition level, the values for PCIMPFLG
are either =1 or =2. (For more details on general data editing/imputation methodology,
please see section 3.0).
CLMOMFLG indicates if a prescription medicine event went through the charge
and payment section of the HC. Prescription medicine events that went through
the charge and payment section of the HC include: (1) events where the person
filed their own prescription claim forms with their insurance company, (2)
events for persons who responded they did not know if they filed their own
prescription claim forms with their insurance company, and (3) insulin and
diabetic supply/equipment events (OMTYPE=2 or =3) that were mentioned in the
Other Medical section of the HC. For these types of events information on payment
sources was retained to the extent that these data were reported by the household
in the charge and payment section of the HC.
INPCFLG denotes whether or not a household respondent had at least one prescription
drug purchase in the PC (0=no, 1=yes).
Return To Table Of Contents
2.7.2.5 The Sample Variable (SAMPLE)
SAMPLE indicates if a respondent reported receiving a free sample of the prescription
medicine in the round (0=no, 1=yes). Each household respondent was asked in
each round whether or not they received any free samples of a reported prescribed
medicine during the round. However, respondents were not asked to report the
number of free samples received, nor was it made clear that free samples were
included in the count of the number of times that the respondent reported purchasing
or otherwise obtaining the prescribed medicine during the round. It is important
for analysts to note, SAMPLE is not a count variable of free samples; SAMPLE
=1 for all acquisitions of a prescribed medicine that a respondent reported
getting a free sample of during the round. This flag variable simply allows
individual analysts to determine for themselves how free samples should be
handled in their analysis.
2.7.2.6 Condition Codes (RXICD1X-RXICD3X) and Clinical Classification Codes
(RXCCC1X-RXCCC3X)
Information on household reported medical conditions associated with each
prescribed medicine event are provided on this file. There are up to three
condition and clinical classification codes listed for each prescribed medicine
event (99.7 percent of prescribed medicine events have 0-3 condition records
linked). To obtain complete information associated with an event, the analyst
must link to the 2008 Medical Conditions File. Details on how to link to the
MEPS 2008 Medical Conditions File are provided in the 2008 Appendix File. The
user should note that due to confidentiality restrictions, provider reported
condition information (for non-prescription medicines events) is not publicly
available. Provider reported condition data (again, for non-prescription medicines
events) can be accessed through the MEPS Data Center only.
The medical conditions reported by the HC respondent were recorded by the
interviewer as verbatim text, which were then coded to fully-specified 2008
ICD-9-CM codes, including medical condition, V codes, and a small number of
E codes, by professional coders. Although codes were verified and error rates
did not exceed 2.5 percent for any coder, analysts should not presume this
level of precision in the data; the ability of household respondents to report
condition data that can be coded accurately should not be assumed. For detailed
information on conditions, please refer to the documentation on the 2008 Medical
Conditions File. For frequencies of conditions by event type, please see the
2008 Appendix File, HC-118I.
The ICD-9-CM condition codes were aggregated into clinically meaningful categories.
These categories, included on the file as RXCCC1X-RXCCC3X, were generated using
Clinical Classification Software (CCS) (formerly known as Clinical Classifications
for Health Care Policy Research (CCHPR)), which aggregates conditions and V-codes
into 263 mutually exclusive categories, most of which are clinically homogeneous.
In order to preserve respondent confidentiality, nearly all of the condition
codes provided on this file have been collapsed from fully-specified codes
to 3-digit code categories. The reported ICD-9-CM code values were mapped to
the appropriate clinical classification category prior to being collapsed to
the 3-digit categories. Because of this collapsing, it is possible for there
to be duplicate 3-digit ICD-9-CM condition codes linked to a single prescribed
medicine event when different fully-specified codes are collapsed into the
same code. This would result in two or more of the condition code variables
on this file being set to the same value on a single record. For more information
on ICD-9-CM codes, see the HC-121 documentation.
The condition codes (and clinical classification codes) linked to each prescribed
medicine event are sequenced in the order in which the conditions were reported
by the household respondent, which was in chronological order of reporting
and not in order of importance or severity. Analysts who use the 2008 Medical
Conditions file in conjunction with this prescribed medicines event file should
note that the conditions on this file are sorted differently than they appear
on the Medical Conditions file.
Return To Table Of Contents 2.7.3 Multum Lexicon Variables from Cerner Multum, Inc.
Each record on this file contains the following Multum Lexicon variables:
PREGCAT - pregnancy category variable - identifies the FDA pregnancy category
to which a particular drug has been assigned
TCn - therapeutic classification variable - assigns a drug to one or more
therapeutic/chemical categories; can have up to three categories per drug
TCnSn - therapeutic sub-classification variable - assigns one or more sub-categories
to a more general therapeutic class category given to a drug
TCnSn_n - therapeutic sub sub-classification variable - assigns one or more
sub sub-categories to a more general therapeutic class category and sub-category
given to a drug
For additional information on these and other Multum Lexicon variables, as
well as the Multum Lexicon database itself, please refer to the following Web
site: http://www.multum.com/Lexicon.htm
Researchers using the Multum Lexicon variables are requested to cite Multum
Lexicon as the data source.
Return To Table Of Contents 2.7.4 Expenditure Variables (RXSF08X-RXXP08X)
2.7.4.1 Definition of Expenditures
Expenditures on this file refer to what is paid for health care services.
More specifically, expenditures in MEPS are defined as the sum of payments
for care received, including out of pocket payments and payments made by private
insurance, Medicaid, Medicare and other sources. The definition of expenditures
used in MEPS differs slightly from its predecessors, the 1987 NMES and 1977
NMCES surveys, where “charges” rather than “sum of payments” were
used to measure expenditures. This change was adopted because charges became
a less appropriate proxy for medical expenditures during the 1990s due to the
increasingly common practice of discounting charges. Although measuring expenditures
as the sum of payments incorporates discounts in the MEPS expenditure estimates,
the estimates do not incorporate any manufacturer or other rebates associated
with Medicaid or other purchases. Another general change from the two prior
surveys is that charges associated with uncollected liability, bad debt, and
charitable care (unless provided by a public clinic or hospital) are not counted
as expenditures, because there are no payments associated with those classifications.
For details on expenditure definitions, please reference the following, “Informing
American Health Care Policy” (Monheit, Wilson, Arnett, 1999).
If examining trends in MEPS expenditures or performing longitudinal analysis
on MEPS expenditures, please refer to section C, sub-section 2.1 for more information.
Return To Table Of Contents 2.7.4.2 Sources of Payment
In addition to total expenditures, variables are provided which itemize expenditures
according to major source of payment categories. These categories are:
- Out-of-pocket by user (self) or family,
- Medicare,
- Medicaid,
- Private Insurance,
- Veterans Administration/CHAMPVA, excluding TRICARE,
- TRICARE,
- Other Federal sources - includes Indian Health Service, Military Treatment
Facilities, and other care by the Federal government,
- Other State and Local Source - includes community and neighborhood
clinics, State and local health departments, and State programs
other than Medicaid,
- Worker’s Compensation, and
- Other Unclassified Sources - includes sources such as
automobile, homeowner’s,
and liability insurances, and other miscellaneous or unknown sources
Two additional source of payment variables were created to classify payments
for events with apparent inconsistencies between insurance coverage and sources
of payment based on data collected in the survey. These variables include:
- Other Private - any type of private insurance payments reported for
persons not reported to have any private health insurance coverage during
the year
as defined in MEPS; and
- Other Public - Medicaid/Medicaid payments reported for persons who
were not reported to be enrolled in the Medicaid/Medicaid program at
any time during
the year
Though relatively small in magnitude, data users/analysts should exercise
caution when interpreting the expenditures associated with these two additional
sources of payment. While these payments stem from apparent inconsistent responses
to health insurance and source of payment questions in the survey, some of
these inconsistencies may have logical explanations. For example, private insurance
coverage in MEPS is defined as having a major medical plan covering hospital
and physician services. If a MEPS sampled person did not have such coverage
but had a single service type insurance plan (e.g., dental insurance) that
paid for a particular episode of care, those payments may be classified as “other
private.” Some of the “other public” payments may stem from
confusion between Medicaid and other state and local programs or may be from
persons who were not enrolled in Medicaid, but were presumed eligible by a
provider who ultimately received payments from the program.
Return To Table Of Contents 2.7.5 Sample Weight (PERWT08F)
2.7.5.1 Overview
There is a single full year person-level weight (PERWT08F) assigned to each
record for each key, in-scope person who responded to MEPS for the full period
of time that he or she was in-scope during 2008. A key person either was a
member of an NHIS household at the time of the NHIS interview, or became a
member of a family associated with such a household after being out-of-scope
at the time of the NHIS (the latter circumstance includes newborns as well
as persons returning from military service, an institution, or living outside
the United States). A person is in-scope whenever he or she is a member of
the civilian noninstitutionalized portion of the U.S. population.
2.7.5.2 Details on Person Weights Construction
The person-level weight PERWT08F was developed in several stages. Person-level
weights for Panels 12 and 13 were created separately. The weighting process
for each panel included an adjustment for nonresponse over time and calibration
to independent population figures. The calibration was initially accomplished
separately for each panel by raking the corresponding sample weights to Current
Population Survey (CPS) population estimates based on five variables. The five
variables used in the establishment of the initial person-level control figures
were: census region (Northeast, Midwest, South, West); MSA status (MSA, non-MSA);
race/ethnicity (Hispanic, non-Hispanic with black as sole reported race, non-Hispanic
with Asian as sole reported race, and other); sex; and age. A 2008 composite
weight was then formed by multiplying each weight from Panel 12 by the factor
.39 and each weight from Panel 13 by the factor .61. The choice of factors
reflected the relative sample sizes of the two panels, helping to limit the
variance of estimates obtained from pooling the two samples. The composite
weight was again raked to the same set of CPS-based control totals. When poverty
status information derived from income variables became available, a final
raking was undertaken on the previously established weight variable. Control
totals were established using poverty status (five categories: below poverty,
from 100 to 125 percent of poverty, from 125 to 200 percent of poverty, from
200 to 400 percent of poverty, at least 400 percent of poverty) as well as
the original five variables used in the previous calibrations.
Return To Table Of Contents 2.7.5.3 MEPS Panel 12 Weight
The person-level weight for MEPS Panel 12 was developed using the 2007 full
year weight for an individual as a “base” weight for survey participants
present in 2007. For key, in-scope respondents who joined an RU some time in
2008 after being out-of-scope in 2007, the 2007 family weight associated with
the family the person joined served as a “base” weight. The weighting
process included an adjustment for nonresponse over Rounds 4 and 5 as well
as raking to population control figures for December 2008. These control figures
were derived by scaling back the population totals obtained from the March
2009 CPS to correspond to a national estimate for the civilian noninstitutionalized
population provided by the Census Bureau for December 2008. Variables used
in the establishment of person-level control figures included: census region
(Northeast, Midwest, South, West); MSA status (MSA, non-MSA); race/ethnicity
(Hispanic, black but non-Hispanic, Asian but non-Hispanic, and other); sex;
and age. Key, responding persons not in-scope on December 31, 2008 but in-scope
earlier in the year retained, as their final Panel 12 weight, the weight after
the nonresponse adjustment.
2.7.5.4 MEPS Panel 13 Weight
The person-level weight for MEPS Panel 13 was developed using the MEPS Round
1 person-level weight as a “base” weight. For key, in-scope respondents
who joined an RU after Round 1, the Round 1 family weight served as a “base” weight.
The weighting process included an adjustment for nonresponse over Round 2 and
the 2008 portion of Round 3 as well as raking to the same population control
figures for December 2008 used for the MEPS Panel 12 weights. The same five
variables employed for Panel 12 raking (census region, MSA status, race/ethnicity,
sex, and age) were used for Panel 13 raking. Similarly, for Panel 13, key,
responding persons not in-scope on December 31, 2008 but in-scope earlier in
the year retained, as their final Panel 13 weight, the weight after the nonresponse
adjustment.
Note that the MEPS Round 1 weights incorporated the following components:
the original household probability of selection for the NHIS; ratio-adjustment
to NHIS-based national population estimates at the household (occupied dwelling
unit) level; adjustment for nonresponse at the dwelling unit level for Round
1; and poststratification to figures at the family and person level obtained
from the March CPS data base of the corresponding year (i.e., 2007 for Panel
12 and 2008 for Panel 13).
Return To Table Of Contents 2.7.5.5 The Final Weight for 2008
The composite weights of two groups of persons who were out-of-scope on December
31, 2008 were poststratified. Specifically, the weights of those who were in-scope
some time during the year, out-of-scope on December 31, and entered a nursing
home during the year were poststratified to a corresponding control total obtained
from the 1996 MEPS Nursing Home Component. Those who died while in-scope during
2008 were poststratified to corresponding estimates derived using data obtained
from the Medicare Current Beneficiary Survey (MCBS) and Vital Statistics information
provided by the National Center for Health Statistics (NCHS). Separate decedent
control totals were developed for the “65 and older” and “under
65” civilian noninstitutionalized populations. The sum of the person-level
weights across all persons assigned a positive person level weight is 304,375,942.
2.7.5.6
Additional Adjustment to 2008 Person Weights for Persons Age 65 and Over
In developing the final 2008 person-level weights (PERWT08F), an adjustment was made at the end of the process to mitigate a noticeable decline in the proportion of elderly persons with at least one hospital stay based on the weight derived using the traditional MEPS weighting methodology. This decline is inconsistent with trends in MEPS and other data sources and the underlying explanation is under investigation. A ratio adjustment strategy was applied only to weights for persons age 65 and over (3,493 persons) and therefore it did not affect the weights for persons under age 65 (29,573 persons). Moreover, the adjustments were carried out by MSA status based on a logistic regression analysis that showed inconsistent changes from 2007 to 2008 in the likelihood of hospitalization for MSA residents versus non-MSA residents. The table below shows the derivations of the adjustment factors that were applied (total of 4 factors) to the previously described final weights.
Ratio Adjustment Factors Applied to Analytic Weight Variable for Persons Age 65 and older
|
# of Hospital Stays (IPDIS08)
|
MSA Resident
|
Non-MSA Resident
|
| 0 |
.808/.845 = 0.9562 |
.808/.780 = 1.0359 |
| 1 or more |
.192/.155 = 1.2387 |
.192/.220 = 0.8727 |
Within each of the 2 MSA subgroups, separate factors were developed for persons with no hospital stays and for persons with at least one stay. The numerators are based on MEPS annual averages of the proportion of elderly persons with at least one hospital stay for the preceding 3 year period (2005-07) and can be regarded as control proportions. The denominators of the factors reflect estimated proportions based on the traditional final weight. For MSA residents, applying these factors to the weights had the joint effect of inflating the estimated proportion of elderly persons with at least one hospital stay while proportionately deflating the proportion with no stays. For non-MSA residents, applying these factors to the weights had the joint effect of deflating the estimated proportion of elderly persons with at least one hospital stay while proportionately inflating the proportion with no stays.
Finally, the weights for the elderly were adjusted by a constant factor to compensate for the negligible impact of rounding on the aggregate weights that resulted from applying the factors in the table above. This factor was derived as the ratio of the sum of weights prior to applying the factors to the sum after applying the factors (39,742,176 / 39,737,780).
Overall, the weighted population estimate for the civilian noninstitutionalized population for December 31, 2008 is 300,257,026 (PERWT08F>0 and INSC1231=1). The sum of the person-level weights across all persons assigned a positive person-level weight is 304,375,942.
2.7.5.7
Coverage
The target population for MEPS in this file is the 2008 U.S. civilian noninstitutionalized population. However, the MEPS sampled households are a subsample of the NHIS households interviewed in 2006 (Panel 12) and 2007 (Panel 13). New households created after the NHIS interviews for the respective Panels and consisting exclusively of persons who entered the target population after 2006 (Panel 12) or after 2007 (Panel 13) are not covered by MEPS. Neither are previously out-of-scope persons who join an existing household but are unrelated to the current household residents. Persons not covered by a given MEPS panel thus include some members of the following groups: immigrants; persons leaving the military; U.S. citizens returning from residence in another country; and persons leaving institutions. The set of uncovered persons constitutes only a small segment of the MEPS target population.
3.0 General Data Editing and Imputation Methodology
The general approach to preparing the household prescription data for this
file was to utilize the PC prescription data to impute information collected
from pharmacy providers to the household drug mentions. For events that went
through the charge and payment section of the HC (events where the person filed
their own prescription claim forms with their insurance company, events for
persons who responded they did not know if they filed their own prescription
claim forms with their insurance company, and insulin and diabetic supply/equipment
events (OMTYPE=2 or =3) that were mentioned in the Other Medical section of
the HC), information on payment sources was retained to the extent that these
data were reported by the household in the charge and payment section of the
HC. A matching program was adopted to link PC drugs and the corresponding drug
information to household drug mentions. To improve the quality of these matches,
all drugs on the household and pharmacy files were coded using a proprietary
database on the basis of the medication names provided by the household and
pharmacy, and, when available, the NDC provided in the pharmacy follow-back
component. The matching process was done at a drug (active ingredient) level,
as opposed to an acquisition level. Considerable editing was done prior to
the matching to correct data inconsistencies in both data sets and to fill
in missing data and correct outliers on the pharmacy file.
Drug price-per-unit outliers were analyzed on the pharmacy file by first identifying
the average wholesale unit price (AWUP) of the drug by linkage through the
NDC to a secondary data file. In general, prescription drug unit prices were
deemed to be outliers by comparing unit prices reported in the pharmacy database
to the AWUP reported in the secondary data file and were edited, as necessary.
Beginning with the 2007 data, the rules used to identify outlier prices for
prescription medications in the PC changed. New outlier thresholds were established
based on the distribution of the ratio of retail unit prices relative to the
AWUP in the 2006 MarketScan Outpatient Pharmaceutical Claims data base. The
new thresholds vary by patent status, whereas in prior years they did not.
These changes improve data quality in three ways: (1) the distribution of prices
in the MEPS better benchmarks to MarketScan, overall and by patent status (Zodet
et al. 2010), (2) fewer pharmacy-reported payments and quantities (for example,
number of pills) are edited, and (3) imputed prices reflect prices paid, rather
than AWUPs. As a result, compared with earlier years of the MEPS, starting
with 2007 there is more variation in prices for generics, lower mean prices
for generics, higher mean prices for brand name drugs, greater differences
in prices between generic and brand name drugs, and a somewhat lower proportion
of spending on drugs by families, as opposed to third-party payers. Beginning
with the 2008 data, pharmacy reports of free antibiotics were not edited as
if they were outliers.
Drug matches between household drug mentions and pharmacy drug events for
a person in the PC were based on drug code, medication name, and the round
in which the drug was reported. The matching of household drug mentions to
pharmacy drugs was performed so that the most detailed and accurate information
for each prescribed medicine event was obtained. Beginning with the 2008 Prescription
Drug file, the criteria for matching were changed to allow multiple NDCs for
the same drug reported by pharmacies (for example, different manufacturers)
to match to one drug reported by the household. Exact dates of purchase were
only available from the follow-back component. The matching program assigned
scores to potential matches. Numeric variables required exact matches to receive
a high score, while partial scores could be assigned to matches between character
variables, such as prescription name, depending on the degree of similarity
in the spelling and sound of the medication names. Household drug mentions
that were deemed exact matches to PC drugs for the same person in the same
round required sufficiently high scores to reflect a high quality match. Initially,
exact matches were used only once and were taken out of the donor pool from
that point on (i.e., these matches were made without replacement). Beginning
with the 2008 Prescription Drug file, however, for remaining persons with pharmacy
data from any round and unmatched household drugs, additional matches are made
with replacement across rounds. Any refill of a household drug mention that
had been matched to a pharmacy drug event was also matched to the same pharmacy
drug event. All remaining unmatched household drug mentions for persons either
in or out of the PC were statistically matched to the entire pharmacy donor
base with replacement by medication name, drug code, type of third party coverage,
health conditions, age, sex, and other characteristics of the individual. Potential
PC donor records were omitted from these matches whenever a NDC was imputed
to the PC record and was not an exact match on a generic product code applied
to all records in the HC and PC. Some matches have inconsistencies between
the PC donor’s potential sources of payment and those of the HC recipient,
and these were resolved. Beginning with the 2008 data, the method used to resolve
inconsistencies in potential payers was changed to better reflect the distribution
of sources of payment among the acquisitions with consistent sources of payment.
This change (1) reduced Medicare payments and increased private payments among
Medicare beneficiaries, and (2) reduced out-of-pocket payments and increased
Medicaid payments among Medicaid enrollees. In addition, Medicare, Medicaid,
and private drug expenditures better benchmark totals in the National Health
Expenditure Accounts.
For more information on the MEPS Prescribed Medicines editing and imputation
procedures, please see J. Moeller, 2001.
Return To Table Of Contents 3.1 Rounding
Expenditure variables on the 2008 Prescribed Medicines file have been rounded
to the nearest penny. Person-level expenditure variables released on the 2008
Full Year Consolidated Data File were rounded to the nearest dollar. It should
be noted that using the 2008 MEPS event files to create person-level totals
will yield slightly different totals than those found on the 2008 Full Year
Consolidated Data File. These differences are due to rounding only. Moreover,
in some instances, the number of persons having expenditures on the 2008 event
files for a particular source of payment may differ from the number of persons
with expenditures on the 2008 Full Year Consolidated Data File for that source
of payment. This difference is also an artifact of rounding only. Please see
the 2008 Appendix File for details on such rounding differences.
3.2 Edited/Imputed Expenditure Variables (RXSF08X-RXXP08X)
There are 13 expenditure variables included on this event file. All of these
expenditures have gone through an editing and imputation process and have been
rounded to the second decimal place. There is a sum of payments variable (RXXP08X)
which for each prescribed medicine event sums all the expenditures from the
various sources of payment. The 12 sources of payment expenditure variables
for each prescribed medicine event are the following: amount paid by self or
family (RXSF08X), amount paid by Medicare (RXMR08X), amount paid by Medicaid
(RXMD08X), amount paid by private insurance (RXPV08X), amount paid by the Veterans
Administration (RXVA08X), amount paid by TRICARE (RXTR08X), amount paid by
other federal sources (RXOF08X), amount paid by state and local (non-federal)
government sources (RXSL08X), amount paid by Worker’s Compensation (RXWC08X),
and amount paid by some other source of insurance (RXOT08X). As mentioned previously,
there are two additional expenditure variables called RXOR08X and RXOU08X (other
private and other public, respectively). These two expenditure variables were
created to maintain consistency between what the household reported as their
private and public insurance status for hospitalization and physician coverage
and third party prescription payments from other private and public sources
(such as a separate private prescription policy or prescription coverage from
the Veterans Administration, the Indian Health Service, or a State assistance
program other than Medicaid). Users should exercise caution when interpreting
the expenditures associated with these two additional sources of payment. While
these payments stem from apparent inconsistent responses to health insurance
and source of payment questions in the survey, some of these inconsistencies
may have logical explanations. Please see section 2.7.4 for details on these
and all other source of payment variables.
Return To Table Of Contents 4.0 Strategies for Estimation
4.1 Developing Event-Level Estimates
The data in this file can be used to develop national 2008 event level estimates
for the U.S. civilian noninstitutionalized population on prescribed medicine
purchases (events) as well as expenditures, and sources of payment for these
purchases. Estimates of total number of purchases are the sum of the weight
variable (PERWT08F) across relevant event records while estimates of other
variables must be weighted by PERWT08F to be nationally representative. The
tables below contain event-level estimates for selected variables.
Selected Event (Purchase) Level Estimates
All Prescribed Medicine Purchases
|
Estimate of Interest
|
Variable Name
|
Estimate (SE)
|
|
Number of purchases (in millions)
|
PERWT08F
|
3149.9 (85.73)
|
|
Mean total payments per purchase
|
RXXP08X
|
$79 (1.1)
|
|
Mean out-of-pocket payment per purchase
|
RXSF08X
|
$20 (0.5)
|
|
Mean proportion of expenditures paid by private insurance
per purchase
|
RXPV08X /RXXP08X
|
0.232 (0.0048)
|
Example by Drug Type: Statins (TC1S1_1=173 or TC1S1_2=173
or TC1S2_1=173 or TC1S3_1 =173 or TC2S1_1=173 or TC2S1_2=173)
|
Estimate of Interest
|
Variable Name
|
Estimate (SE)
|
|
Number of purchases (in millions)
|
PERWT08F
|
205.2 (7.48)
|
|
Mean total payments per purchase
|
RXXP08X
|
$102 (2.3)
|
|
Mean annual total payments per person
|
RXXP08X (aggregated across purchases within person)
|
$606 (13.8)
|
Example by Associated Condition: Hypertension (RXICD1X=”401” or
RXICD2X=”401” or RXICD3X=”401”)
|
Estimate of Interest
|
Variable Name
|
Estimate (SE)
|
|
Number of purchases (in millions)
|
PERWT08F
|
504.1 (16.73)
|
|
Mean total payments per purchase
|
RXXP08X
|
$42 (0.9)
|
|
Mean annual total payments per person
|
RXXP08X (aggregated across purchases within person)
|
$407 (9.6)
|
Return To Table Of Contents
4.2 Person-Based Estimates for Prescribed Medicine Purchases
To enhance analyses of prescribed medicine purchases, analysts may link information
about prescribed medicine purchases by sample persons in this file to the annual
full year consolidated file (which has data for all MEPS sample persons), or
conversely, link person-level information from the full year consolidated file
to this event level file (see section 5 below for more details). Both this
file and the Full Year Consolidated File may be used to derive estimates for
persons with prescribed medicine purchases and annual estimates of total expenditures
for these purchases. However, if the estimate relates to the entire population,
this file cannot be used to calculate the denominator, as only those persons
with at least one prescribed medicine purchase are represented on this data
file. Therefore, the full year consolidated file must be used for person-level
analyses that include both persons with and without prescribed medicine events.
4.3 Variables with Missing Values
It is essential that the analyst examine all variables for the presence of
negative values used to represent missing values. For continuous or discrete
variables, where means or totals may be taken, it may be necessary to set negative
values to values appropriate to the analytic needs. That is, the analyst should
either impute a value or set the value to one that will be interpreted as missing
by the computing language used. For categorical and dichotomous variables,
the analyst may want to consider whether to recode or impute a value for cases
with negative values or whether to exclude or include such cases in the numerator
and/or denominator when calculating proportions.
Methodologies used for the editing/imputation of expenditure variables (e.g.,
total expenditures and sources of payment) are described in Section 3.2.
Return To Table Of Contents 4.4 Variance Estimation (VARSTR, VARPSU)
MEPS has a complex sample design. To obtain estimates of variability (such
as the standard error of sample estimates or corresponding confidence intervals)
for MEPS estimates, analysts need to take into account the complex sample design
of MEPS for both person-level and family-level analyses. Several methodologies
have been developed for estimating standard errors for surveys with a complex
sample design, including the Taylor-series linearization method, balanced repeated
replication, and jackknife replication. Various software packages provide analysts
with the capability of implementing these methodologies. Replicate weights
have not been developed for the MEPS data. Instead, the variables needed to
calculate appropriate standard errors based on the Taylor-series linearization
method are included on this file as well as all other MEPS public use files.
Software packages that permit the use of the Taylor-series linearization method
include SUDAAN, Stata, SAS (version 8.2 and higher), and SPSS (version 12.0
and higher). For complete information on the capabilities of each package,
analysts should refer to the corresponding software user documentation.
Using the Taylor-series linearization method, variance estimation strata and
the variance estimation PSUs within these strata must be specified. The variance
strata variable is named VARSTR, while the variance PSU variable is named VARPSU.
Specifying a “with replacement” design in a computer software package,
such as SUDAAN, provides standard errors appropriate for assessing the variability
of MEPS survey estimates. It should be noted that the number of degrees of
freedom associated with estimates of variability indicated by such a package
may not appropriately reflect the actual number available. For MEPS sample
estimates for characteristics generally distributed throughout the country
(and thus the sample PSUs), one can expect at least 100 degrees of freedom
for the 2008 full year data associated with the corresponding estimates of
variance and usually substantially more.
Prior to 2002, MEPS variance strata and PSUs were developed independently
from year to year, and the last two characters of the strata and PSU variable
names denoted the year. However, beginning with the 2002 Point-in-Time PUF,
the variance strata and PSUs were developed to be compatible with MEPS data
associated with the NHIS sample design used through 2006. Such data can be
pooled and the variance strata and PSU variables provided can be used without
modification for variance estimation purposes for estimates covering multiple
years of data.
As a result of the change in the NHIS sample design in 2006, a new set of
variance strata and PSUs have been established for variance estimation
purposes for
use with MEPS Panel 12 and subsequent MEPS panels. There were 165 variance
strata associated with both MEPS Panel 12 and Panel 13, providing a substantial
number of degrees of freedom for subgroups as well as the nation as a whole.
Each variance stratum contains either two or three variance estimation PSUs.
Return To Table Of Contents
5.0 Merging/Linking MEPS Data Files
Data from this file can be used alone or in conjunction with other files for
different analytic purposes. This section summarizes various scenarios for
merging/linking MEPS event files. Each MEPS panel can also be linked back to
the previous years’ National Health Interview Survey public use data
files. For information on obtaining MEPS/NHIS link files please see www.meps.ahrq.gov/data_stats/more_info_download_data_files.jsp.
5.1 Linking to the Person-Level File
Merging characteristics of interest from the person-level file (e.g., MEPS
2008 Full Year Consolidated File) expands the scope of potential estimates.
For example, to estimate the total number of prescribed medicine purchases
of persons with specific demographic characteristics (such as age, race, sex,
and education), population characteristics from a person-level file need to
be merged onto the prescribed medicines file. This procedure is illustrated
below. The MEPS 2008 Appendix File, HC-118I, provides additional detail on
how to merge MEPS data files.
- Create data set PERSX by sorting the 2008 Full Year Consolidated File
by the person identifier, DUPERSID. Keep only variables to be merged onto
the prescribed medicines file and DUPERSID.
- Create data set PMEDS by sorting the 2008 Prescribed Medicines File
by person identifier, DUPERSID.
- Create final data set NEWPMEDS by merging these two files by DUPERSID,
keeping only records on the prescribed medicines file.
The following is an example of SAS code, which completes these steps:
PROC SORT DATA=IN.HC121 (KEEP=DUPERSID AGE31X AGE42X AGE53X SEX RACEX EDUCYR)
OUT=PERSX;
BY DUPERSID;
RUN;
PROC SORT DATA=IN.HC118A
OUT=PMEDS;
BY DUPERSID;
RUN;
DATA NEWPMEDS;
MERGE PMEDS (IN=A) PERSX (IN=B);
BY DUPERSID;
IF A;
RUN;
Return To Table Of Contents
5.2 Linking to the Medical Conditions File
The CLNK provides a link from MEPS event files to the 2008 Medical Conditions
File. When using the CLNK, data users/analysts should keep in mind that (1)
conditions are self-reported, (2) there may be multiple conditions associated
with a prescribed medicine purchase, and (3) a condition may link to more than
one prescribed medicine purchase or any other type of purchase. Users should
also note that not all prescribed medicine purchases link to the condition
file.
5.3 Pooling Annual Files
To facilitate analysis of subpopulations and/or low prevalence events, it
may be desirable to pool together more than one year of data to yield sample
sizes large enough to generate reliable estimates. For more details on pooling
MEPS data files see www.meps.ahrq.gov/data_stats/download_data_files_detail.jsp?cboPufNumber=HC-036.
www.meps.ahrq.gov/data_stats/download_data_files_detail.jsp?cboPufNumber=HC-036.
Starting in Panel 9, values for DUPERSID from previous panels will occasionally
be re-used. Therefore, it is necessary to use the panel variable (PANEL) in
combination with DUPERSID to ensure unique person-level identifiers across
panels. Creating unique records in this manner is advised when pooling MEPS
data across multiple annual files that have one or more identical values for
DUPERSID.
5.4 Longitudinal Analysis
Panel-specific files containing estimation variables to facilitate longitudinal
analysis are available for downloading in the data section of the MEPS Web
site.
Return To Table Of Contents
References
Cohen, S.B. (1998). Sample Design of the 1996 Medical Expenditure Panel Survey
Medical Provider Component. Journal of Economic and Social Measurement. Vol
24, 25-53.
Cohen, S.B. (1997). Sample Design of the 1996 Medical Expenditure Panel
Survey Household Component. Rockville (MD): Agency for Health Care Policy and Research;
1997. MEPS Methodology Report, No. 2. AHCPR Pub. No. 97-0027.
Cohen, J.W. (1997). Design and Methods of the Medical Expenditure Panel
Survey Household Component. Rockville (MD): Agency for Health Care Policy and Research;
1997. MEPS Methodology Report, No. 1. AHCPR Pub. No. 97-0026.
Cohen, S.B. (1996). The Redesign of the Medical Expenditure Panel Survey:
A Component of the DHHS Survey Integration Plan. Proceedings of the COPAFS
Seminar on Statistical Methodology in the Public Service.
Cox, B.G. and Cohen, S.B. (1985). Chapter 8: Imputation Procedures to Compensate
for Missing Responses to Data Items. In Methodological Issues
for Health Care Surveys. Marcel Dekker, New York.
Moeller J.F., Stagnitti, M., Horan, E., et al. Outpatient Prescription
Drugs: Data Collection and Editing in the 1996 Medical Expenditure Panel
Survey (HC-010A). Rockville (MD): Agency for Healthcare Research and Quality; 2001. MEPS Methodology
Report No. 12. AHRQ Pub. No. 01-0002.
Monheit, A.C., Wilson, R., and Arnett, III, R.H. (Editors). Informing
American Health Care Policy. (1999). Jossey-Bass Inc, San Francisco.
Shah, B.V., Barnwell, B.G., Bieler, G.S., Boyle, K.E., Folsom, R.E., Lavange,
L., Wheeless, S.C., and Williams, R. (1996). Technical Manual: Statistical
Methods and Algorithms Used in SUDAAN Release 7.0, Research Triangle Park,
NC: Research Triangle Institute.
Zodet, M., Hill, S., and Miller, G.E. “Comparison of Retail Drug
Prices in the MEPS and MarketScan: Implications for MEPS Editing Rules.” Agency
for Healthcare Research and Quality Working Paper, 2009. Return To Table Of Contents
D. Variable-Source Crosswalk
MEPS HC-118A: 2008 Prescribed Medicines Events
Survey Administration Variables
|
Variable
|
Description
|
Source
|
|
DUID
|
Dwelling unit ID
|
Assigned in sampling
|
|
PID
|
Person number
|
Assigned in sampling
|
|
DUPERSID
|
Sample person ID (DUID + PID)
|
Assigned in sampling
|
|
RXRECIDX
|
Record ID – Unique Prescribed Medicine Identifier
|
Constructed
|
|
LINKIDX
|
Link to condition and other event files
|
CAPI derived
|
|
PANEL
|
Panel indicator
|
Assigned in sampling
|
|
PURCHRD
|
Round in which the Rx/prescribed medicine was obtained/purchased
|
CAPI derived
|
|
DUP2007
|
Duplicate acquisition on 2007 PMED file
|
Constructed
|
Return To Table Of Contents
Prescribed Medicines Events Variables
|
Variable
|
Description
|
Source
|
|
RXBEGMM
|
Month person first used medicine
|
PM11OV2
|
|
RXBEGYRX
|
Year person first used medicine
|
PM11
|
|
RXNAME
|
Medication name (Imputed)
|
Imputed
|
|
RXNDC
|
National drug code (Imputed)
|
Imputed
|
|
RXQUANTY
|
Quantity of Rx/prescribed medicine (Imputed)
|
Imputed
|
|
RXFORM
|
Form of Rx/prescribed medicine (Imputed)
|
Imputed
|
|
RXFRMUNT
|
Unit of measurement for form of Rx/prescribed
medicine (Imputed)
|
Imputed
|
|
RXSTRENG
|
Strength of Rx/prescribed medicine dose (Imputed)
|
Imputed
|
|
RXSTRUNT
|
Unit of measurement for strength of Rx/prescribed medicine
dose (Imputed)
|
Imputed
|
|
PHARTP1-PHARTP17
|
Type of pharmacy provider – (1st-17th)
|
PM16
|
|
RXFLG
|
Flag variable indicating imputation source for NDC on
pharmacy donor record
|
Constructed
|
|
PCIMPFLG
|
Flag indicating type of household to pharmacy prescription
match
|
Constructed
|
|
CLMOMFLG
|
Charge/payment, Rx claim filing, and OMTYPE =2 or =3
(insulin and diabetic supply equipment events) status
|
CP01/Constructed
|
|
INPCFLG
|
Flag indicating if the person has at least one record
in the pharmacy component
|
Constructed
|
|
SAMPLE
|
Flag indicating if a respondent received a free sample
of this drug in the round
|
CAPI derived
|
|
RXICD1X
|
3 digit ICD-9 condition code
|
PM09
|
|
RXICD2X
|
3 digit ICD-9 condition code
|
PM09
|
|
RXICD3X
|
3 digit ICD-9 condition code
|
PM09
|
|
RXCCC1X
|
Modified Clinical Classification Code
|
Constructed/Edited
|
|
RXCCC2X
|
Modified Clinical Classification Code
|
Constructed/Edited
|
|
RXCCC3X
|
Modified Clinical Classification Code
|
Constructed/Edited
|
|
PREGCAT
|
Multum Pregnancy Category
|
Cerner Multum, Inc.
|
|
TC1
|
Multum Therapeutic Class #1
|
Cerner Multum, Inc.
|
|
TC1S1
|
Multum Therapeutic Sub-Class #1 for TC1
|
Cerner Multum, Inc.
|
|
TC1S1_1
|
Multum Therapeutic Sub-Sub-Class for TC1S1
|
Cerner Multum, Inc.
|
|
TC1S1_2
|
Multum Therapeutic Sub-Sub-Class for TC1S1
|
Cerner Multum, Inc.
|
|
TC1S2
|
Multum Therapeutic Sub-Class #2 for TC1
|
Cerner Multum, Inc.
|
|
TC1S2_1
|
Multum Therapeutic Sub-Sub-Class for TC1S2
|
Cerner Multum, Inc.
|
|
TC1S3
|
Multum Therapeutic Sub-Class #3 for TC1
|
Cerner Multum, Inc.
|
|
TC1S3_1
|
Multum Therapeutic Sub-Sub-Class for TC1S3
|
Cerner Multum, Inc.
|
|
TC2
|
Multum Therapeutic Class #2
|
Cerner Multum, Inc.
|
|
TC2S1
|
Multum Therapeutic Sub-Class #1 for TC2
|
Cerner Multum, Inc.
|
|
TC2S1_1
|
Multum Therapeutic Sub-Sub-Class for TC2S1
|
Cerner Multum, Inc.
|
|
TC2S1_2
|
Multum Therapeutic Sub-Sub-Class for TC2S1
|
Cerner Multum, Inc.
|
|
TC2S2
|
Multum Therapeutic Sub-Class #2 for TC2
|
Cerner Multum, Inc.
|
|
TC3
|
Multum Therapeutic Class #3
|
Cerner Multum, Inc.
|
|
TC3S1
|
Multum Therapeutic Sub-Class #1 for TC3
|
Cerner Multum, Inc.
|
|
TC3S1_1
|
Multum Therapeutic Sub-Sub-Class for TC3S1
|
Cerner Multum, Inc.
|
|
RXSF08X
|
Amount paid, self or family (Imputed)
|
CP11/Edited/Imputed
|
|
RXMR08X
|
Amount paid, Medicare (Imputed)
|
CP12/CP13/Edited/Imputed
|
|
RXMD08X
|
Amount paid, Medicaid (Imputed)
|
CP12/CP13/Edited/Imputed
|
|
RXPV08X
|
Amount paid, private insurance (Imputed)
|
CP12/CP13/Edited/Imputed
|
|
RXVA08X
|
Amount paid, Veteran’s Administration (Imputed)
|
CP12/CP13/Edited/Imputed
|
|
RXTR08X
|
Amount paid, TRICARE (Imputed)
|
CP12/CP13/Edited/Imputed
|
|
RXOF08X
|
Amount paid, other Federal (Imputed)
|
CP12/CP13/Edited/Imputed
|
|
RXSL08X
|
Amount paid, state and local government (Imputed)
|
CP12/CP13/Edited/Imputed
|
|
RXWC08X
|
Amount paid, Worker’s Compensation (Imputed)
|
CP12/CP13/Edited/Imputed
|
|
RXOT08X
|
Amount paid, other insurance (Imputed)
|
CP12/CP13/Edited/Imputed
|
|
RXOR08X
|
Amount paid, other private (Imputed)
|
Constructed/Imputed
|
|
RXOU08X
|
Amount paid, other public (Imputed)
|
Constructed/Imputed
|
|
RXXP08X
|
Sum of payments RXSF08X – RXOU08X (Imputed)
|
CP12/CP13/Edited/Imputed
|
Return To Table Of Contents
Weights
|
Variable
|
Description
|
Source
|
|
PERWT08F
|
Poverty/mortality/nursing home adjusted person-level
weight
|
Constructed
|
|
VARSTR
|
Variance estimation stratum, 2008
|
Constructed
|
|
VARPSU
|
Variance estimation PSU, 2008
|
Constructed
|
Return To Table Of Contents
Attachment 1
Definitions of Abbreviations for RXFORM
|
Dosage Form
|
Definition
|
|
-7
|
refused
|
|
-8
|
don’t know
|
|
-9
|
not ascertained
|
|
ACC
|
accessory
|
|
ADR
|
acetic acid drop
|
|
AE
|
aerosol
|
|
AER
|
aerosol
|
|
AER SPRAY
|
aerosol spray
|
|
AERO
|
aerosol
|
|
AEROSOL
|
|
|
AMP
|
ampule
|
|
ARO
|
aerosol solid
|
|
AUTO INJ
|
auto-injection
|
|
BACK SUPPORT BELT
|
|
|
BAG
|
|
|
BAL
|
balm
|
|
BALM
|
|
|
BAN
|
bandage
|
|
BANDAGE
|
|
|
BAR
|
|
|
BATTERY
|
|
|
BENCH
|
|
|
BOT
|
bottle
|
|
BOTTLE
|
|
|
BOX
|
|
|
BOXES
|
|
|
BRACE
|
|
|
BRIEF
|
|
|
BUT
|
butterfly
|
|
C
|
capsules, or cream (varies)
|
|
C12
|
12 hour extended-release capsule
|
|
C24
|
24 hour extended-release capsule
|
|
CA
|
capsule
|
|
CANE
|
|
|
CAP
|
capsule
|
|
CAP DR
|
delayed-release capsule
|
|
CAP ER
|
extended-release capsule
|
|
CAP SA
|
slow-acting capsule
|
|
CAPLET
|
|
|
CAPLT
|
caplet
|
|
CAPS
|
capsules
|
|
CAPSULE
|
|
|
CAPSULE SA
|
slow-acting capsule
|
|
CATHETER
|
|
|
CC
|
cubic centimeter
|
|
CER
|
extended-release capsule
|
|
CHAMBER
|
|
|
CHEW
|
chewable tablet
|
|
CHEW TAB
|
chewable tablet
|
|
CHEW TABS
|
chewable tablets
|
|
CHEWABLE
|
|
|
CHW
|
chewable tablets
|
|
CLEANSER
|
|
|
COLLAR
|
|
|
COMBO
|
|
|
COMPOUND
|
|
|
CON
|
condom
|
|
CONDOM
|
|
|
CONTAINER
|
|
|
COTTON
|
|
|
CPSR
|
slow-release capsule
|
|
CR
|
cream
|
|
CRE
|
cream
|
|
CREA
|
cream
|
|
CREAM
|
|
|
CRM
|
cream
|
|
CRY
|
crystal
|
|
CRYSTAL
|
|
|
CTB
|
chewable tablets
|
|
CTG
|
cartridge
|
|
CUTTER
|
|
|
DEV
|
device
|
|
DEVICE
|
|
|
DIA
|
diaper
|
|
DIAPER
|
|
|
DIAPHRAM
|
|
|
DIS
|
disk, or dermal infusion system
|
|
DISK
|
|
|
DOS PAK
|
dose pack
|
|
DR
|
drop
|
|
DRE
|
dressing
|
|
DRESSING
|
|
|
DRO
|
drop
|
|
DROP
|
|
|
DROPS
|
|
|
DROPS OPTH OTI
|
ophthalmic/otic drops
|
|
DROPS SUSP
|
drops suspension
|
|
DRP
|
drop
|
|
DRPS
|
drops
|
|
DSK
|
disk
|
|
DSPK
|
tablets in a dose pack
|
|
EAR DROP
|
|
|
EAR DROPS
|
|
|
EAR DRP
|
ear drop
|
|
EAR SUSP
|
ear suspension
|
|
EC TABS
|
enteric coated tablets
|
|
ECC
|
enteric coated capsules
|
|
ECT
|
enteric coated tablets
|
|
ELI
|
elixir
|
|
ELIX
|
elixir
|
|
ELIXIR
|
|
|
ELX
|
elixir
|
|
EMERGENCY KIT
|
|
|
EMO
|
emollient
|
|
EMU
|
emulsion
|
|
EMULSION
|
|
|
ENEMA
|
|
|
ERTA
|
extended-release tablets
|
|
EXTN CAP
|
extended-release capsule
|
|
EXTRACT
|
|
|
EYE DRO
|
eye drop
|
|
EYE DROP
|
|
|
EYE DROPS
|
|
|
EYE DRP
|
eye drop
|
|
EYE SO
|
eye solution
|
|
FIL
|
film
|
|
FILM ER
|
film, extended-release
|
|
FILMTAB
|
|
|
FILMTABS
|
|
|
FLOWMETER
|
|
|
FOA
|
foam
|
|
FOAM
|
|
|
GAU
|
gauze
|
|
GAUZE
|
|
|
GEF
|
effervescent granules
|
|
GEL
|
|
|
GEL CAP
|
gel capsule
|
|
GER
|
|
|
GFS
|
gel-forming solution
|
|
GLOVE
|
|
|
GRA
|
granules
|
|
GRANULES
|
|
|
GRR
|
grams
|
|
GTT
|
drops
|
|
GUM
|
|
|
HOSE
|
medical hosiery
|
|
HU
|
capsule
|
|
ICR
|
control-release insert
|
|
IMPLANT
|
|
|
IN
|
injectible
|
|
INH
|
inhalant
|
|
INH AER
|
inhalant aerosol
|
|
INHAL
|
inhalant
|
|
INHAL SOL
|
inhalant solution
|
|
INHALER
|
|
|
INHL
|
inhalant
|
|
INJ
|
injectible
|
|
INJECTION (S)
|
|
|
INSERT
|
|
|
INSULIN
|
|
|
IUD
|
intrauterine devise
|
|
IV
|
intravenous
|
|
JEL
|
jelly
|
|
JELLY
|
|
|
KIT
|
|
|
L
|
lotion
|
|
LANCET
|
|
|
LANCET (S)
|
|
|
LI
|
liquid
|
|
LINIMENT
|
|
|
LIQ
|
liquid
|
|
LIQUID
|
|
|
LOLLIPOP
|
|
|
LOT
|
lotion
|
|
LOTION
|
|
|
LOZ
|
lozenge
|
|
LOZENGE
|
|
|
MASK
|
|
|
MCG
|
microgram
|
|
METER
|
|
|
MG
|
milligram
|
|
MIS
|
miscellaneous
|
|
MIST
|
|
|
MONITOR
|
|
|
MOUTHWASH
|
|
|
NAS
|
nasal spray
|
|
NASAL
|
|
|
NASAL INHALER
|
|
|
NASAL POCKET HL
|
nasal inhaler, pocket
|
|
NASAL SOLN
|
nasal solution
|
|
NASAL SPR
|
nasal spray
|
|
NASAL SPRAY
|
|
|
NDL
|
needle
|
|
NE
|
nebulizer
|
|
NEB
|
nebulizer
|
|
NEBULIZER
|
|
|
NEEDLE
|
|
|
NEEDLES
|
|
|
NMA
|
enema
|
|
NMO
|
nanomole, millimicromole
|
|
ODR
|
ophthalmic drop (ointment)
|
|
ODT
|
oral disintegrating tablet
|
|
OIL
|
|
|
OIN
|
ointment
|
|
OINT
|
ointment
|
|
OINT TOP
|
topical ointment
|
|
OINTMENT
|
|
|
ONT
|
ointment
|
|
OP
|
ophthalmic solution
|
|
OP DROPS
|
ophthalmic drops
|
|
OP SOL
|
ophthalmic solution
|
|
OPH
|
ophthalmic
|
|
OPH S
|
ophthalmic solution or suspension
|
|
OPH SOL
|
ophthalmic solution
|
|
OPH SOLN
|
ophthalmic solution
|
|
OPHT SOL
|
ophthalmic solution
|
|
OPHTH DROP (S)
|
ophthalmic drops
|
|
OPHTH OINT
|
ophthalmic ointment
|
|
OPHTH SOLN
|
ophthalmic solution
|
|
OPT SLN
|
ophthalmic solution
|
|
OPT SOL
|
ophthalmic solution
|
|
OPTH
|
ophthalmic solution or suspension or ointment
|
|
OPTH S
|
ophthalmic solution or suspension
|
|
OPTH SLN
|
ophthalmic solution
|
|
OPTH SOL
|
ophthalmic solution
|
|
OPTH SUSP
|
ophthalmic suspension
|
|
OPTIC
|
|
|
ORAL
|
|
|
ORAL INHL
|
oral inhalant
|
|
ORAL INHALER
|
|
|
ORAL PWD
|
oral powder
|
|
ORAL RINSE
|
|
|
ORAL SOL
|
oral solution
|
|
ORAL SUS
|
oral suspension
|
|
ORAL SUSP
|
oral suspension
|
|
OTI
|
otic solution
|
|
OTIC
|
|
|
OTIC SOL
|
otic solution
|
|
OTIC SOLN
|
otic solution
|
|
OTIC SUS
|
otic suspension
|
|
OTIC SUSP
|
otic suspension
|
|
PA
|
tablet pack, pad or patch (varies)
|
|
PAC
|
pack
|
|
PACK
|
|
|
PAD
|
|
|
PADS
|
|
|
PAK
|
pack
|
|
PAS
|
paste
|
|
PASTE
|
|
|
PAT
|
patch
|
|
PATCH
|
|
|
PEN
|
|
|
PCH
|
patch
|
|
PDR
|
powder
|
|
PDS
|
powder for reconstitution
|
|
PEDIATRIC DROPS
|
|
|
PI1
|
powder for injection, 1 month
|
|
PI3
|
powder for injection, 3 months
|
|
PIH
|
powder for inhalation
|
|
PKG
|
package
|
|
PKT
|
packet
|
|
PLASTER
|
|
|
PLEDGETS
|
|
|
PO-SYRUP
|
syrup by mouth (oral syrup)
|
|
POPSICLE
|
|
|
POUCH
|
|
|
POW
|
powder
|
|
POWD
|
powder
|
|
POWDER
|
|
|
POWDER/SUSPENS
|
powder/suspension
|
|
PRO
|
prophylactic
|
|
PST
|
paste
|
|
PULVULE
|
|
|
PWD
|
powder
|
|
PWD F/SOL
|
powder for solution
|
|
RCTL SUPP
|
rectal suppository
|
|
RECTAL CREAM
|
|
|
REDITABS
|
|
|
REF
|
|
|
RIN
|
Rinse
|
|
RING
|
|
|
RINSE
|
|
|
ROLL
|
|
|
S
|
syrup, suspension, solution (varies)
|
|
SA CAPS
|
Slow-acting capsules
|
|
SA TAB
|
Slow-acting tablet
|
|
SA TABLETS
|
Slow-acting tablets
|
|
SA TABS
|
Slow-acting tablets
|
|
SAL
|
Salve
|
|
SCRUB
|
|
|
SER
|
extended-release suspension
|
|
SET
|
|
|
SGL
|
soft b23gel cap
|
|
SHA
|
shampoo
|
|
SHAM
|
shampoo
|
|
SHMP
|
shampoo
|
|
SHOE
|
|
|
SLT
|
sublingual tablet
|
|
SL TAB
|
sublingual tablet
|
|
SO
|
solution
|
|
SOA
|
Soap
|
|
SOL
|
solution
|
|
SOLN
|
solution
|
|
SOLUTION
|
|
|
SOLU
|
solution
|
|
SP
|
spray
|
|
SPG
|
sponge
|
|
SPN
|
|
|
SPONGE
|
|
|
SPR
|
spray
|
|
SPRAY
|
|
|
SRN
|
syringe
|
|
STOCKING
|
|
|
STP
|
Strip
|
|
STR
|
Strip
|
|
STRIP
|
|
|
STRIPS
|
|
|
SU
|
suspension, solution,
suppository, powder, or
granules
for reconstitution (varies)
|
|
SUB
|
sublingual
|
|
SUP
|
suppository
|
|
SUPP
|
suppository
|
|
SUPPOSITORIES
|
|
|
SUPPOSITORY
|
|
|
SUS
|
suspension
|
|
SUS/LIQ
|
suspension/liquid
|
|
SUSP
|
suspension
|
|
SUSPEN
|
suspension
|
|
SUSPENDED RELEASE CAPLET
|
|
|
SUSPENSION
|
|
|
SWA
|
Swab
|
|
SWAB
|
|
|
SWABS
|
|
|
SYP
|
syrup
|
|
SYR
|
syrup
|
|
SYRG
|
syringe
|
|
SYRINGE
|
|
|
SYRP
|
syrup
|
|
SYRUP
|
|
|
T
|
tablet
|
|
T12
|
12 hour extended-release tablet
|
|
T24
|
24 hour extended-release tablet
|
|
TA
|
tablet
|
|
TAB
|
tablet
|
|
TAB CHEW
|
chewable tablet
|
|
TAB DR
|
delayed-release tablet
|
|
TAB EC
|
enteric coated tablet
|
|
TAB SL
|
Slow-acting tablet
|
|
TAB SUBL
|
sublingual tablet
|
|
TABL
|
tablet
|
|
TABLET
|
|
|
TABLET CUTTER
|
|
|
TABLET SPLITTER
|
|
|
TABLETS
|
|
|
TABS
|
tablets
|
|
TAP
|
Tape
|
|
TAPE
|
|
|
TB
|
tablet
|
|
TBCH
|
chewable tablet
|
|
TBS
|
tablets
|
|
TBSL
|
sublingual tablet
|
|
TBSR
|
Slow-release tablet
|
|
TCP
|
tablet, coated particles
|
|
TDM
|
extended-release film
|
|
TDR
|
orally disintegrating tablets
|
|
TDS
|
transdermal system
|
|
TEF
|
effervescent tablet
|
|
TER
|
extended-release tablet
|
|
TES
|
Test
|
|
TEST
|
|
|
TEST STRIP
|
|
|
TEST STRIPS
|
|
|
TIN
|
tincture
|
|
TOP CREAM
|
topical cream
|
|
TOP OINT
|
topical ointment
|
|
TOP SOL
|
topical solution
|
|
TOP SOLN
|
topical solution
|
|
TOPICAL
|
|
|
TOPICAL CREAM
|
|
|
TOPICAL SOLUTION
|
|
|
TRO
|
troche
|
|
TTB
|
Time release tablet
|
|
TUB
|
Tube
|
|
TUBE
|
|
|
UNDERWEAR
|
|
|
UNIT DOSE
|
|
|
UNT
|
Unit
|
|
VAGINAL CREAM
|
|
|
VAPORIZER
|
|
|
VIA
|
Vial
|
|
VIAL
|
|
|
VIAL(S)
|
|
|
VIL
|
Vial
|
|
WAF
|
wafer
|
|
WALKER
|
|
|
WASH
|
|
|
WIPES
|
|
|
Z-PAK
|
|
Return To Table Of Contents Attachment 2
Definitions of Codes and Abbreviations for RXFRMUNT
Attachment 3
Definitions of Abbreviations, Codes and Symbols for RXSTRUNT
|
Abbreviations, Codes and Symbols
|
Definition
|
|
-7
|
refused
|
|
-8
|
don't know
|
|
-9
|
not ascertained
|
|
%
|
percent
|
|
09
|
compound
|
|
ACTIVATION
|
activation
|
|
ACTUATION
|
actuation
|
|
CC
|
cubic centimeters
|
|
CM2
|
square centimeter
|
|
DOSE
|
dose
|
|
DRP
|
drop
|
|
EL
|
ELISA (enzyme linked immunosorbent assay)
|
|
G
|
gram
|
|
GM
|
gram
|
|
GR
|
grain
|
|
HR or HRS
|
hour, hours
|
|
INH
|
inhalation
|
|
IU
|
international unit
|
|
MCG
|
microgram
|
|
MEQ
|
microequivalent
|
|
MG
|
milligram
|
|
ML
|
milliliter
|
|
MMU
|
millimass units
|
|
OZ
|
ounce
|
|
PACKET
|
packet
|
|
PFU
|
plaque forming units
|
|
SQ CM
|
square centimeter
|
|
U
|
units
|
Return To Table Of Contents
Attachment 4
Theraputic Class Code Definitions
|
Theraputic Class Code
|
Definition
|
|
1
|
anti-infectives
|
|
2
|
amebicides
|
|
3
|
anthelmintics
|
|
4
|
antifungals
|
|
5
|
antimalarial agents
|
|
6
|
antituberculosis agents
|
|
7
|
antiviral agents
|
|
8
|
carbapenems
|
|
9
|
cephalosporins
|
|
10
|
leprostatics
|
|
11
|
macrolide derivatives
|
|
12
|
miscellaneous antibiotics
|
|
13
|
penicillins
|
|
14
|
quinolones
|
|
15
|
sulfonamides
|
|
16
|
tetracyclines
|
|
17
|
urinary anti-infectives
|
|
18
|
aminoglycosides
|
|
19
|
antihyperlipidemic agents
|
|
20
|
antineoplastics
|
|
21
|
alkylating agents
|
|
22
|
antineoplastic antibiotics
|
|
23
|
antimetabolites
|
|
24
|
antineoplastic hormones
|
|
25
|
miscellaneous antineoplastics
|
|
26
|
mitotic inhibitors
|
|
27
|
radiopharmaceuticals
|
|
28
|
biologicals
|
|
30
|
antitoxins and antivenins
|
|
31
|
bacterial vaccines
|
|
32
|
colony stimulating factors
|
|
33
|
immune globulins
|
|
34
|
in vivo diagnostic biologicals
|
|
36
|
recombinant human erythropoietins
|
|
37
|
toxoids
|
|
38
|
viral vaccines
|
|
39
|
miscellaneous biologicals
|
|
40
|
cardiovascular agents
|
|
41
|
agents for hypertensive emergencies
|
|
42
|
angiotensin converting enzyme inhibitors
|
|
43
|
antiadrenergic agents, peripherally acting
|
|
44
|
antiadrenergic agents, centrally acting
|
|
45
|
antianginal agents
|
|
46
|
antiarrhythmic agents
|
|
47
|
beta-adrenergic blocking agents
|
|
48
|
calcium channel blocking agents
|
|
49
|
diuretics
|
|
50
|
inotropic agents
|
|
51
|
miscellaneous cardiovascular agents
|
|
52
|
peripheral vasodilators
|
|
53
|
vasodilators
|
|
54
|
vasopressors
|
|
55
|
antihypertensive combinations
|
|
56
|
angiotensin II inhibitors
|
|
57
|
central nervous system agents
|
|
58
|
analgesics
|
|
59
|
miscellaneous analgesics
|
|
60
|
narcotic analgesics
|
|
61
|
nonsteroidal anti-inflammatory agents
|
|
62
|
salicylates
|
|
63
|
analgesic combinations
|
|
64
|
anticonvulsants
|
|
65
|
antiemetic/antivertigo agents
|
|
66
|
antiparkinson agents
|
|
67
|
anxiolytics, sedatives, and hypnotics
|
|
68
|
barbiturates
|
|
69
|
benzodiazepines
|
|
70
|
miscellaneous anxiolytics, sedatives and hypnotics
|
|
71
|
CNS stimulants
|
|
72
|
general anesthetics
|
|
73
|
muscle relaxants
|
|
74
|
neuromuscular blocking agents
|
|
76
|
miscellaneous antidepressants
|
|
77
|
miscellaneous antipsychotic agents
|
|
79
|
psychotherapeutic combinations
|
|
80
|
miscellaneous central nervous system agents
|
|
81
|
coagulation modifiers
|
|
82
|
anticoagulants
|
|
83
|
antiplatelet agents
|
|
84
|
heparin antagonists
|
|
85
|
miscellaneous coagulation modifiers
|
|
86
|
thrombolytics
|
|
87
|
gastrointestinal agents
|
|
88
|
antacids
|
|
89
|
anticholinergics/antispasmodics
|
|
90
|
antidiarrheals
|
|
91
|
digestive enzymes
|
|
92
|
gallstone solubilizing agents
|
|
93
|
GI stimulants
|
|
94
|
H2 antagonists
|
|
95
|
laxatives
|
|
96
|
miscellaneous GI agents
|
|
97
|
hormones/hormone modifiers
|
|
98
|
adrenal cortical steroids
|
|
99
|
antidiabetic agents
|
|
100
|
miscellaneous hormones
|
|
101
|
sex hormones
|
|
102
|
contraceptives
|
|
103
|
thyroid hormones
|
|
104
|
immunosuppressive agents
|
|
105
|
miscellaneous agents
|
|
106
|
antidotes
|
|
107
|
chelating agents
|
|
108
|
cholinergic muscle stimulants
|
|
109
|
local injectable anesthetics
|
|
110
|
miscellaneous uncategorized agents
|
|
111
|
psoralens
|
|
112
|
radiocontrast agents
|
|
113
|
genitourinary tract agents
|
|
114
|
illicit (street) drugs
|
|
115
|
nutritional products
|
|
116
|
iron products
|
|
117
|
minerals and electrolytes
|
|
118
|
oral nutritional supplements
|
|
119
|
vitamins
|
|
120
|
vitamin and mineral combinations
|
|
121
|
intravenous nutritional products
|
|
122
|
respiratory agents
|
|
123
|
antihistamines
|
|
124
|
antitussives
|
|
125
|
bronchodilators
|
|
126
|
methylxanthines
|
|
127
|
decongestants
|
|
128
|
expectorants
|
|
129
|
miscellaneous respiratory agents
|
|
130
|
respiratory inhalant products
|
|
131
|
antiasthmatic combinations
|
|
132
|
upper respiratory combinations
|
|
133
|
topical agents
|
|
134
|
anorectal preparations
|
|
135
|
antiseptic and germicides
|
|
136
|
dermatological agents
|
|
137
|
topical anti-infectives
|
|
138
|
topical steroids
|
|
139
|
topical anesthetics
|
|
140
|
miscellaneous topical agents
|
|
141
|
topical steroids with anti-infectives
|
|
143
|
topical acne agents
|
|
144
|
topical antipsoriatics
|
|
146
|
mouth and throat products
|
|
147
|
ophthalmic preparations
|
|
148
|
otic preparations
|
|
149
|
spermicides
|
|
150
|
sterile irrigating solutions
|
|
151
|
vaginal preparations
|
|
153
|
plasma expanders
|
|
154
|
loop diuretics
|
|
155
|
potassium-sparing diuretics
|
|
156
|
thiazide diuretics
|
|
157
|
carbonic anhydrase inhibitors
|
|
158
|
miscellaneous diuretics
|
|
159
|
first generation cephalosporins
|
|
160
|
second generation cephalosporins
|
|
161
|
third generation cephalosporins
|
|
162
|
fourth generation cephalosporins
|
|
163
|
ophthalmic anti-infectives
|
|
164
|
ophthalmic glaucoma agents
|
|
165
|
ophthalmic steroids
|
|
166
|
ophthalmic steroids with anti-infectives
|
|
167
|
ophthalmic anti-inflammatory agents
|
|
168
|
ophthalmic lubricants and irrigations
|
|
169
|
miscellaneous ophthalmic agents
|
|
170
|
otic anti-infectives
|
|
171
|
otic steroids with anti-infectives
|
|
172
|
miscellaneous otic agents
|
|
173
|
HMG-CoA reductase inhibitors
|
|
174
|
miscellaneous antihyperlipidemic agents
|
|
175
|
protease inhibitors
|
|
176
|
NRTIs
|
|
177
|
miscellaneous antivirals
|
|
178
|
skeletal muscle relaxants
|
|
179
|
skeletal muscle relaxant combinations
|
|
180
|
adrenergic bronchodilators
|
|
181
|
bronchodilator combinations
|
|
182
|
androgens and anabolic steroids
|
|
183
|
estrogens
|
|
184
|
gonadotropins
|
|
185
|
progestins
|
|
186
|
sex hormone combinations
|
|
187
|
miscellaneous sex hormones
|
|
191
|
narcotic analgesic combinations
|
|
192
|
antirheumatics
|
|
193
|
antimigraine agents
|
|
194
|
antigout agents
|
|
195
|
5HT3 receptor antagonists
|
|
196
|
phenothiazine antiemetics
|
|
197
|
anticholinergic antiemetics
|
|
198
|
miscellaneous antiemetics
|
|
199
|
hydantoin anticonvulsants
|
|
200
|
succinimide anticonvulsants
|
|
201
|
barbiturate anticonvulsants
|
|
202
|
oxazolidinedione anticonvulsants
|
|
203
|
benzodiazepine anticonvulsants
|
|
204
|
miscellaneous anticonvulsants
|
|
205
|
anticholinergic antiparkinson agents
|
|
206
|
miscellaneous antiparkinson agents
|
|
208
|
SSRI antidepressants
|
|
209
|
tricyclic antidepressants
|
|
210
|
phenothiazine antipsychotics
|
|
211
|
platelet aggregation inhibitors
|
|
212
|
glycoprotein platelet inhibitors
|
|
213
|
sulfonylureas
|
|
214
|
biguanides
|
|
215
|
insulin
|
|
216
|
alpha-glucosidase inhibitors
|
|
217
|
bisphosphonates
|
|
218
|
alternative medicines
|
|
219
|
nutraceutical products
|
|
220
|
herbal products
|
|
222
|
penicillinase resistant penicillins
|
|
223
|
antipseudomonal penicillins
|
|
224
|
aminopenicillins
|
|
225
|
beta-lactamase inhibitors
|
|
226
|
natural penicillins
|
|
227
|
NNRTIs
|
|
228
|
adamantane antivirals
|
|
229
|
purine nucleosides
|
|
230
|
aminosalicylates
|
|
231
|
nicotinic acid derivatives
|
|
232
|
rifamycin derivatives
|
|
233
|
streptomyces derivatives
|
|
234
|
miscellaneous antituberculosis agents
|
|
235
|
polyenes
|
|
236
|
azole antifungals
|
|
237
|
miscellaneous antifungals
|
|
238
|
antimalarial quinolines
|
|
239
|
miscellaneous antimalarials
|
|
240
|
lincomycin derivatives
|
|
241
|
fibric acid derivatives
|
|
242
|
psychotherapeutic agents
|
|
243
|
leukotriene modifiers
|
|
244
|
nasal lubricants and irrigations
|
|
245
|
nasal steroids
|
|
246
|
nasal antihistamines and decongestants
|
|
247
|
nasal preparations
|
|
248
|
topical emollients
|
|
249
|
antidepressants
|
|
250
|
monoamine oxidase inhibitors
|
|
251
|
antipsychotics
|
|
252
|
bile acid sequestrants
|
|
253
|
anorexiants
|
|
254
|
immunologic agents
|
|
256
|
interferons
|
|
257
|
immunosuppressive monoclonal antibodies
|
|
261
|
heparins
|
|
262
|
coumarins and indandiones
|
|
263
|
impotence agents
|
|
264
|
urinary antispasmodics
|
|
265
|
urinary pH modifiers
|
|
266
|
miscellaneous genitourinary tract agents
|
|
267
|
ophthalmic antihistamines and decongestants
|
|
268
|
vaginal anti-infectives
|
|
269
|
miscellaneous vaginal agents
|
|
270
|
antipsoriatics
|
|
271
|
thiazolidinediones
|
|
272
|
proton pump inhibitors
|
|
273
|
lung surfactants
|
|
274
|
cardioselective beta blockers
|
|
275
|
non-cardioselective beta blockers
|
|
276
|
dopaminergic antiparkinsonism agents
|
|
277
|
5-aminosalicylates
|
|
278
|
cox-2 inhibitors
|
|
279
|
gonadotropin-releasing hormone and analogs
|
|
280
|
thioxanthenes
|
|
281
|
neuraminidase inhibitors
|
|
282
|
meglitinides
|
|
283
|
thrombin inhibitors
|
|
284
|
viscosupplementation agents
|
|
285
|
factor Xa inhibitors
|
|
286
|
mydriatics
|
|
287
|
ophthalmic anesthetics
|
|
288
|
5-alpha-reductase inhibitors
|
|
289
|
antihyperuricemic agents
|
|
290
|
topical antibiotics
|
|
291
|
topical antivirals
|
|
292
|
topical antifungals
|
|
293
|
glucose elevating agents
|
|
295
|
growth hormones
|
|
296
|
inhaled corticosteroids
|
|
297
|
mucolytics
|
|
298
|
mast cell stabilizers
|
|
299
|
anticholinergic bronchodilators
|
|
300
|
corticotropin
|
|
301
|
glucocorticoids
|
|
302
|
mineralocorticoids
|
|
303
|
agents for pulmonary hypertension
|
|
304
|
macrolides
|
|
305
|
ketolides
|
|
306
|
phenylpiperazine antidepressants
|
|
307
|
tetracyclic antidepressants
|
|
308
|
SSNRI antidepressants
|
|
309
|
miscellaneous antidiabetic agents
|
|
310
|
echinocandins
|
|
311
|
dibenzazepine anticonvulsants
|
|
312
|
cholinergic agonists
|
|
313
|
cholinesterase inhibitors
|
|
314
|
antidiabetic combinations
|
|
315
|
glycylcyclines
|
|
316
|
cholesterol absorption inhibitors
|
|
317
|
antihyperlipidemic combinations
|
|
318
|
insulin-like growth factor
|
|
319
|
vasopressin antagonists
|
|
320
|
smoking cessation agents
|
|
321
|
ophthalmic diagnostic agents
|
|
322
|
ophthalmic surgical agents
|
|
324
|
antineoplastic interferons
|
|
325
|
sclerosing agents
|
|
327
|
antiviral combinations
|
|
328
|
antimalarial combinations
|
|
329
|
antituberculosis combinations
|
|
330
|
antiviral interferons
|
|
331
|
radiologic agents
|
|
332
|
radiologic adjuncts
|
|
333
|
miscellaneous iodinated contrast media
|
|
334
|
lymphatic staining agents
|
|
335
|
magnetic resonance imaging contrast media
|
|
336
|
non-iodinated contrast media
|
|
337
|
ultrasound contrast media
|
|
338
|
diagnostic radiopharmaceuticals
|
|
339
|
therapeutic radiopharmaceuticals
|
|
340
|
aldosterone receptor antagonists
|
|
341
|
atypical antipsychotics
|
|
342
|
renin inhibitors
|
|
344
|
nasal anti-infectives
|
|
345
|
fatty acid derivative anticonvulsants
|
|
346
|
gamma-aminobutyric acid reuptake inhibitors
|
|
347
|
gamma-aminobutyric acid analogs
|
|
348
|
triazine anticonvulsants
|
|
349
|
carbamate anticonvulsants
|
|
350
|
pyrrolidine anticonvulsants
|
|
351
|
carbonic anhydrase inhibitor anticonvulsants
|
|
352
|
urea anticonvulsants
|
|
353
|
anti-angiogenic ophthalmic agents
|
|
354
|
H. pylori eradication agents
|
|
355
|
functional bowel disorder agents
|
|
356
|
serotoninergic neuroenteric modulators
|
|
357
|
growth hormone receptor blockers
|
|
358
|
metabolic agents
|
|
359
|
peripherally acting antiobesity agents
|
|
360
|
lysosomal enzymes
|
|
361
|
miscellaneous metabolic agents
|
|
362
|
chloride channel activators
|
|
363
|
probiotics
|
|
364
|
antiviral chemokine receptor antagonist
|
|
365
|
medical gas
|
|
366
|
integrase strand transfer inhibitor
|
|
368
|
non-ionic iodinated contrast media
|
|
369
|
ionic iodinated contrast media
|
|
370
|
otic steroids
|
|
371
|
dipeptidyl peptidase 4 inhibitors
|
|
372
|
amylin analogs
|
|
373
|
incretin mimetics
|
|
374
|
cardiac stressing agents
|
|
375
|
peripheral opioid receptor antagonists
|
|
376
|
radiologic conjugating agents
|
|
377
|
prolactin inhibitors
|
|
378
|
drugs used in alcohol dependence
|
|
379
|
next generation cephalosporins
|
|
380
|
topical debriding agents
|
|
381
|
topical depigmenting agents
|
|
382
|
topical antihistamines
|
|
383
|
antineoplastic detoxifying agents
|
|
384
|
platelet-stimulating agents
|
|
385
|
group I antiarrhythmics
|
|
386
|
group II antiarrhythmics
|
|
387
|
group III antiarrhythmics
|
|
388
|
group IV antiarrhythmics
|
|
389
|
group V antiarrhythmics
|
|
390
|
hematopoietic stem cell mobilizer
|
|
392
|
otic anesthetics
|
|
393
|
cerumenolytics
|
|
394
|
topical astringents
|
|
395
|
topical keratolytics
|
|
396
|
prostaglandin D2 antagonists
|
|
397
|
multikinase inhibitors
|
|
398
|
BCR-ABL tyrosine kinase inhibitors
|
|
399
|
CD52 monoclonal antibodies
|
|
400
|
CD33 monoclonal antibodies
|
|
401
|
CD20 monoclonal antibodies
|
|
402
|
VEGF/VEGFR inhibitors
|
|
403
|
mTOR inhibitors
|
|
404
|
EGFR inhibitors
|
|
405
|
HER2 inhibitors
|
|
406
|
glycopeptide antibiotics
|
|
407
|
inhaled anti-infectives
|
|
408
|
histone deacetylase inhibitors
|
|
409
|
bone resorption inhibitors
|
|
410
|
adrenal corticosteroid inhibitors
|
|
411
|
calcitonin
|
|
412
|
uterotonic agents
|
|
413
|
antigonadotropic agents
|
|
414
|
antidiuretic hormones
|
|
415
|
miscellaneous bone resorption inhibitors
|
|
416
|
somatostatin and somatostatin analogs
|
|
417
|
selective estrogen receptor modulators
|
|
418
|
parathyroid hormone and analogs
|
|
419
|
gonadotropin-releasing hormone antagonists
|
|
420
|
antiandrogens
|
|
422
|
antithyroid agents
|
|
423
|
aromatase inhibitors
|
|
424
|
estrogen receptor antagonists
|
|
426
|
synthetic ovulation stimulants
|
|
427
|
tocolytic agents
|
|
428
|
progesterone receptor modulators
|
|
429
|
trifunctional monoclonal antibodies
|
|
430
|
anticholinergic chronotropic agents
|
|
431
|
anti-CTLA-4 monoclonal antibodies
|
Return To Table Of Contents
|
